Abstract
The study focuses on validating and applying a Monte Carlo (MC) simulation model to backscatter calculations from the shielding discs used during intraoperative electron radiotherapy (IOERT), particularly in breast cancer treatments. The MC model is developed based on dosimetric data collected under reference conditions and validated by measurements with EBT4 Gafchromic films in a water phantom. The study investigates the dose distributions for 6, 9, and 12 MeV electron beams formed by a mobile AQURE accelerator, comparing scenarios with and without a surgical stainless steel shielding disc. While the shielding disc effectively reduces radiation doses behind it, the backscatter significantly increases doses in tissues immediately in front of the disc. Specifically, the dose at 1 mm in front of the disc increases by 19.8%, 18.4%, and 17.5% were observed for 6, 9, and 12 MeV beams, respectively. The validated MC model provides an accurate tool for predicting dose distributions in complex geometries, enabling improved treatment planning and safety in IOERT applications. The findings underscore the need to consider backscatter effects when shielding discs are used in IOERT. The study suggests further optimization of shielding disc design, potentially incorporating biocompatible, low-Z materials to mitigate backscatter.
Similar content being viewed by others
Introduction
Currently, two dedicated intraoperative electron radiation therapy (IOERT) accelerators are available: the Mobetron (IntraOP, Sunnyvale, CA, USA) and the LIAC HWL (Sordina IORT Technologies, Vicenza, Italy)1,2. A third accelerator, known as AQURE, is being developed by the National Centre for Nuclear Research (NCNR, Świerk, Poland)3. Our previous paper detailed this machine’s commissioning, dosimetric characterisation, and performance4.
The IOERT treatment planning systems are designed to be straightforward. During the commissioning of a specific IOERT machine, dosimetric data for all potential electron beams is gathered. Using this data in conjunction with the specific anatomical details of the tissues encountered during surgery, appropriate beam parameters can be selected, facilitating the execution of radiotherapy during the operation5. However, it’s important to conduct additional pre-treatment measurements to ensure dosimetric accuracy for non-standard geometries. To simplify and reduce all further measurements, we have developed the Monte Carlo (MC) simulation model6.
Against the MC models used by other authors (e.g. Alhamada et al.7 or Russo et al.8), our model uses energy spectra reconstructed from dosimetric data obtained under reference conditions, i.e. from the percentage dose distributions (PDD) gathered in a homogeneous environment for beams formed by a 10 cm diameter applicator. The model is designed to simulate PDD for beams formed by applicators with diameters other than 10 cm and in non-homogeneous environments. While we previously demonstrated the model’s accuracy in simulating PDD for varying applicator diameters in a homogeneous environment9 (i.e. for standard geometry conditions), this study showcases the model’s effectiveness in simulations conducted in a non-homogeneous environment (i.e. for non-standard geometry conditions). A key scenario we explored is the frequently used protocol for breast treatment by IOERT, where a shielding disc is implemented to minimise radiation doses to the underlying bone structures (i.e. ribs) and lungs beneath the surgical field (i.e. breast) from which the neoplastic lesion is extracted10. While the dose reduction to the tissues behind the shielding disc is apparent, the impact of backscattered radiation from the disc on the values of doses deposited in tissues located directly in front of the disc depends significantly on its design, material composition, and the energy of the electron beam7,8.
Therefore, in this study, we validated via gafchromic measurements the accuracy of our MC model in non-standard geometry conditions, exploring the value of backscattered radiation from a surgical stainless steel shielding disc for IOERT realised by the AQURE mobile accelerator.
Materials and methods
Dose measurements
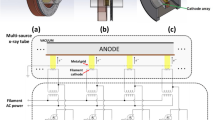
A 20 × 30 × 20 cm3 water phantom was fabricated to accommodate a holder for a gafchromic film, a shielding disc, and an applicator fixation (Fig. 1a and b). The grey part of the holder allows placing the gafchromic film parallel to the beam axis and fixing the shielding disc at a precisely defined depth. The film positioning using the holder is fraught with 2 mm uncertainty. The purple part fixes the applicator, allowing the applicator and the shielding disc to be maintained mutually coaxially.
Specific information about phantom components:
-
(1)
Phantom holder: Original Prusa i3 MK3S + 3D printer (Prusa Research; Prague, Czech Republic) was used to print the phantom holder by fused deposition modelling technology with polylactic acid filament11.
-
(2)
Shielding disc: the disc made of surgical stainless steel (316 L alloy12), 7 cm in diameter and 0.5 cm thick.
-
(3)
Film: Self-developing EBT4 Gafchromic films (Ashland Inc. Bridgewater, NJ, USA). The EBT4 films are near tissue equivalent, have high spatial resolution (25 μm or less), enable non-uniformity correction using multi-channel dosimetry, and decrease UV/visible light sensitivity compared to previous generations. The film’s response is independent of temperature (up to 600C), atmospheric pressure, and the direction of the irradiation beam. These films allow us to measure the doses (optimal range from 0.2 to 10 Gy) obtained by radiation beams with energies ranging from 100 keV to 18 MeV13.
We measured PDD for the 6 MeV, 9 MeV, and 12 MeV electron beams formed by the 6 cm diameter applicator hard docked to the AQURE mobile accelerator (NCNR, Świerk, Poland)14. The applicator was fixed perpendicular to the water in the phantom, with its tip touching the water’s surface. Two measurement geometries were used. The first one included the shielding disc placed on the R90 depth: 19, 27, and 31 mm for 6, 9, and 12 MeV electron beams, respectively4. The disc was positioned parallel to the water surface and aligned coaxially with the electron beam. The EBT4 films were placed along the central axis of the beam (CAX), positioned above (from the water’s surface to the top of the disc) and below (from the bottom surface of the disc to the phantom base) the shielding disc (Fig. 1d). The second setup, which does not include the shielding disc, employs a single sheet of EBT4 film placed in the CAX from the water’s surface to the phantom base (Fig. 1c). Films were irradiated with a dose of 9 Gy defined at the points of maximum dose located below the water surface at the depths of 11, 16, and 18 mm for 6, 9, and 12 MeV electron beams, respectively4.
The calibration procedure of the EBT4 films
For the dose calibration, the film was cut into pieces (3 × 3 cm2) and irradiated with an electron beam (20 × 20 cm2, 9 MeV, TrueBeam, Varian Medical Systems, USA) in ranges from 1 to 12 Gy. The film was positioned in a water-equivalent solid phantom, where the dose was measured by the ion chamber. The film readout procedure for calibration and measurements was done 36 h after irradiation, using the flatbed scanner Epson Perfection 850 Pro scanner (Seiko Epson Corporation, Japan) with a 4 mm glass plate over the film and with the following parameters: scan resolution 300 dpi, no colour correction, transmission mode, 48-bit Red-Green-Blue (RGB), and saved in the uncompressed TIFF data format. Scans were analysed using Mephysto mc2, Film Analyze 1.8 (PTW Freiburg, Freiburg, Germany; single red channel analysis from RGB). For such a calibration procedure, the reading of doses from the film used further in the experiment is subject to 2% uncertainty.
(a) Shielding disc (steel circle) and the holder elements: grey for gafchromic film and shielding disc fixation and purple for fixing the applicator, (b) The setup of the holder with the shielding disc in the phantom, (c) Measurement geometry without the shielding disc, (d) Measurement geometry with the shielding disc.
Monte Carlo simulations
The model of the MC simulation was created in the Geant4 v.11.01.p01 package15. Based on the technical documentation of the AQURE mobile accelerator, the exact geometry of the beam-forming system was used3 with previously reconstructed energy spectra for 6, 9, and 12 MeV electron beams. The energy spectra were previously reconstructed using the Dual Annealing method with Tikhonov regularization6, for which mono-energetic depth dose distributions for a 10 cm diameter applicator were used as input data9 (the description of the method is included in the appendix). The spectra that were reconstructed for the applicator with a diameter of 10 cm were validated for other sizes of the applicator, including the applicator with a diameter of 6 cm9.
The aim of this study is to validate the accuracy of our model for non-standard geometries. Therefore, the phantom system and both geometries used during the measurements (with and without a disc) were reproduced and included in the MC simulation model. The geometry with the shielding disc represents non-standard geometry. The detectors were placed in the water phantom in the beam axis at 1 mm intervals from the water surface to a depth of 10 cm. Each detector had a 1 mm thickness in the direction of propagation of the beam. In the case of the simulation with the shielding disc, the detectors were placed in the same way, except for the disc area. The phantom was placed so that its surface was in contact with the end of the applicator. As a result, for each of the three nominal energies (i.e. 6, 9 and 12 MeV) of the electron beam formed by the 6 cm diameter applicator, the PDD distributions were determined twice – for geometry with and without the shielding disc. The number of simulated primary electrons emitted from the source (exit window of the accelerator) for each simulation was 1010. The energy spectra of the primary electrons were determined in the previous study6. The cut-off for secondary produced particles was set to 0.1 mm. Simulations were carried out in parallel on five computers (dual Intel Xeon E5 2.4 GHz, 32 GB RAM each). Figure 2 shows the exact geometry of the beam-forming system and the phantom with the location of the detectors used for MC simulations and the shielding disc (the case of the non-standard geometry).
Data analysis
In the first step, the simulated and the measured PDDs were compared by analysing the absolute point-to-point difference between the doses and the gamma analysis method16. The comparisons were performed for both scenarios – when the PPDs were obtained for geometry with and without the shielding disc.
The gamma analysis was performed in global mode with the criteria of 2% as the acceptable dose difference (DD) and 2 mm as the acceptable distance to agreement (DTA). The analyses were performed without any dose threshold to enable analysis in the contamination area of the PDD curves where the doses are lower than 1% of the maximum dose.
The measurement validation of the MC simulation allows for a direct comparison of the simulated PDDs between the scenarios with and without the shielding disc. The dose difference between the scenarios with and without shielding permits the establishment of the effect of the shield on the dose distribution.
The dose differences (DD) at depth (d) were defined as:
where PDDMC, disc and PDDMC are the values from the percentage depth doses at the same depth (d) obtained respectively from the simulations with and without the shielding disc.
Results
The percentage depth doses for scenarios without and with the shielding disc are shown respectively in Fig. 3a and c. The absolute differences between simulated and measured doses ranged up to 2.5%. The mean absolute dose differences with corresponding standard deviations for the geometry scenario without a shielding disc were 1.2 ± 0.5%, 0.9 ± 0.4% and 1.2 ± 0.6% for the 6, 9, and 12 MeV electron beams, respectively. For the geometry scenario with the shielding disc, the differences were lower and were, respectively, 0.5 ± 0.4%, 0.5 ± 0.3%, and 0.7 ± 0.5% for the 6, 9, and 12 MeV electron beams. However, it should be noted that the positioning of the gafchromic film and the reading of the dose measured by the film are subject to an uncertainty of 2 mm and 2%, respectively. Therefore, the gamma analysis was used as a main method to validate agreement between simulated and measured doses (Fig. 3b and d). The results of gamma analysis confirm agreement between simulated and measured doses. The inconsistencies between measured and simulated doses were found only in points placed directly in front of the shielding disc (Fig. 3d) due to cut-edge errors in the measurements taken at the border of the gafchromic films that affect the dose reading.
The comparisons of the simulated (line) and measured (dots) percentage dose distributions for the geometry scenarios without the shielding disc (a) and with the shielding disc (c) with particular emphasis on the results of the gamma analysis performed in a global mode (b and d). The gamma analysis criteria are 2%/2 mm.
In the second step, to determine the values of backscatter doses, we compared the simulated doses for scenarios with and without the shielding disc (Fig. 4a). When the shielding disc is used, the relative increase in the doses at the point of 1 mm in front of the shielding disc is 19.8%, 18.4%, and 17.5% for 6, 9, and 12 MeV electron beams, respectively (Fig. 4b). The comparison of the simulated doses also confirms the efficacy of the reduction by the shielding disc of the doses deposited behind it, as Fig. 4b shows.
Discussion
The study was realised on the AQURE machine, the mobile linear accelerator designed by the Polish National Centre for Nuclear Research (NCNR) for IOERT purposes. Currently, AQURE is obtaining an EU certificate for clinical use. Due to our hospital’s partnership with NCNR, we had the opportunity to conduct detailed functional usability tests of the AQURE prototype. The results of the measurements that were described in our previous paper (i.e. Ryczkowski et al.4) confirm the dosimetric accuracy of the AQURE accelerator under the literature guidelines and confirm that the functionality of this machine is similar to other machines available in clinical use (i.e. to the Mobetron or LIAC HWL)5,17,18.
Accurate treatment planning for IOERT before surgery can be difficult due to various factors. Typically, monitor units are calculated for a homogeneous environment using simple formulas that do not account for tissue inhomogeneities, surface irregularity or elements such as shielding discs. The dose measurements within the patient’s body during IOERT are limited. Since IOERT is performed in a single session, any deviations that cause incorrect dose distribution cannot be corrected afterwards. Using a shielding disc during breast IOERT can alter the dose distribution due to backscattered electrons. When the presence of the disc leads to difficulties in accurately estimating the dose, MC simulation is often considered the optimal solution8. A comparison of the data obtained from our MC simulation model with the measurement data confirms its accuracy. Due to the 2 mm uncertainty related to the film positioning in the self-constructed holder and the uncertainty of the dose readout from the film, which is 2%, the direct dose comparisons were supported by the gamma analysis method. While the positional uncertainty was established experimentally, the dose readout uncertainty was based on literature19,20. There are no clear recommendations concerning the gamma criteria for IOERT. The AAPM TG-218 report21 focuses on dynamic techniques, for which the general recommendation is to use 3%/2 mm criteria. Nevertheless, the report suggests that the gamma criteria could be more rigorous for the other techniques where other clinical goals are needed. Therefore, we have chosen a 2%/2 mm criteria, which is more stringent than recommended in the TG-218 report and covers the measurement uncertainties well. Costa et al.22 chose the same criteria combination and suggested that 2%/2 mm is the most appropriate for measurement examinations in IOERT. The positive validation result allows us to use our model to estimate the backscattered doses on the shielding disc. Moreover, the model can be used further, without confirmation by measurement, for calculating the doses for any other scenarios when, for example, the structure or position of the shielding disc will be changed.
The usage of the shielding disc during IOERT for breast cancer is still controversial due to the possibility of inaccuracies in positioning7,8,23, which is not the scope of this study. Nevertheless, if the therapeutic team decides to use it, the disc should be properly located in the operative area. Placing the disc at the R90 depth is directly derived from the clinical assumptions, according to which the 90% isodose should cover the treated area with a dose prescription at dmax5,24. An ideal scenario assumes delivery of the treated area with a homogeneous, prescribed dose. It is impossible due to the tissue inhomogeneities in the irradiated area and the typical method of IOERT realisation (i.e., single electron beam). Therefore, physicists and radiotherapists should pay special attention to the doses cumulated in the parts of the treated area near the tip of the applicator (in the dose build-up region) and on the doses near the shielding disc, where backscatter dosage from the disc is observed.
The underdosing near the tip of the applicator can be effectively compensated using biocompatible boluses25. The doses in the target area near the shielding disc are not obvious and should be verified for each beam energy and for every disk material. Our findings show that using surgical stainless steel shielding discs increases the doses at 1 mm in front of the disc by almost 20% compared to the doses that were measured in the same place in the scenario without using the disc. The dose increase depends on the energy of the electron beam, i.e. the lowest and the highest dose increase were noted, respectively, for 12 MeV (17.5%) and 6 MeV (19.8%) energy beams. As a result, the doses for the 6, 9 and 12 MeV electron beams, at 1 mm in front of the disc, are 9.8%, 8.4% and 7.5% higher than the dmax doses where they are normalised (100%). While for the IOERT, the ratio of side effects is relatively low, the dose increment in front of the shielding disc may increase the likelihood of grade 3 or 4 fibrosis, especially when the volume of the irradiated tissues is significant26,27. Therefore, our internal IOERT protocols assume that the dose delivered to the treated area, starting from the entrance surface and ending with the areas located near the shielding disc, should range from 95 to 105% of the prescribed dose. Obtaining knowledge about the actual dose distribution, i.e. the underdosing in the build-up area compensated by the bolus usage as well as the overdosage near shielding disc caused by backscattered radiations, allows clinicians to customise the dose prescription to specified isodose (not to the point, e.g. dmax) ranged from 100 to 90%28.
While the dose increasements directly in front of the disc, in relation to the dmax doses, are below 10%, our findings gave us rationales to further research on reducing the value of backscatter dose. The findings of Alhamada et al.7 suggest that the increment of the backscattered doses positively correlated with the atomic number (Z) of the material from which the disks are constructed. Mihailescu and Botezatu29 show an effective reduction of the backscatter doses when a two-layered (aluminium and lead) shielding disc is used instead of a one-layer (lead) disc. Similar findings show Catalano et al.30 while researching the backscatter led by differently composed layers of shielding discs. Therefore, we believe it is possible to redesign the shielding disc by adding a biocompatible and low Z material placed in front of the steel layer to reduce the value of the backscatter doses leads from the steel layer. The thickness of this layer will be estimated through our validated MC model and verified by phantom measurements in further study.
Conclusion
The validation procedure confirms the accuracy of our MC simulation model in non-standard conditions. Implementing single-layered stainless steel shielding discs resulted in a noticeable increase in the doses deposited at 1 mm in front of the disc by 19.8%, 18.4%, and 17.5% for the 6, 9, and 12 MeV beam, respectively, compared to the doses measured at the same location without the disc.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Meurk, M. L., Goer, D. A., Spalek, G. & Cook, T. The Mobetron: A new concept for IORT. Front. Radiat. Ther. Oncol. 31, 65–70. https://doi.org/10.1159/000061147 (1997).
Winkler, P., Odreitz-Stark, S., Haas, E., Thalhammer, M. & Partl, R. Commissioning, dosimetric characterisation and machine performance assessment of the LIAC HWL mobile accelerator for intraoperative radiotherapy. Z. Med. Phys. 30(4), 279–288. https://doi.org/10.1016/j.zemedi.2020.06.004 (2020).
Adrich, P. et al. A new mobile electron accelerator for intraoperative electron radiation therapy. In Int. J. Mod. Phys. Conf. Ser. Vol. 27, 1460125. https://doi.org/10.1142/S2010194514601252 (2014).
Ryczkowski, A. et al. Commissioning, dosimetric characterisation and machine performance assessment of the AQURE mobile accelerator for intraoperative radiotherapy. Pol. J. Med. Phys. Eng. 30(3), 177–181. https://doi.org/10.2478/pjmpe-2024-0021 (2024).
Beddar, A. S. et al. Intraoperative radiation therapy using mobile electron linear accelerators: Report of AAPM radiation therapy committee task group 72. Med. Phys. 33(5), 1476–1489. https://doi.org/10.1118/1.2194447 (2006).
Ryczkowski, A., Piotrowski, T., Staszczak, M., Wiktorowicz, M. & Adrich, P. Optimization of the regularization parameter in the dual annealing method used for the reconstruction of energy spectrum of electron beam generated by the AQURE mobile accelerator. Z. Med. Phys. 34(4), 510–520. https://doi.org/10.1016/j.zemedi.2023.03.003 (2024).
Alhamada, H. et al. Shielding disk position in intra-operative electron radiotherapy (IOERT): A Monte Carlo study. Phys. Med. 51, 1–6. https://doi.org/10.1016/j.ejmp.2018.05.023 (2018).
Russo, G. et al. Dose distribution changes with shielding disc misalignments and wrong orientations in breast IOERT: A Monte Carlo—GEANT4 and experimental study. J. Appl. Clin. Med. Phys. 13(5), 3817. https://doi.org/10.1120/jacmp.v13i5.3817 (2012).
Ryczkowski, A. et al. Implementation and validation of the method for the energy spectra reconstruction of the electron beams generated by the AQURE mobile accelerator. Rep. Pract. Oncol. Radiother. https://doi.org/10.5603/rpor.104511 (2025).
Kaiser, J. et al. Intraoperative tumour bed boost with electrons in breast cancer of clinical stages I through III: Updated 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 102(1), 92–101. https://doi.org/10.1016/j.ijrobp.2018.05.028 (2018).
Bielęda, G., Marach, A., Boehlke, M., Zwierzchowski, G. & Malicki, J. 3D-printed surface applicators for brachytherapy: A Phantom study. J. Contemp. Brachyther. 13(5), 549–562. https://doi.org/10.5114/jcb.2021.110304 (2021).
Aderibigbe, B. A. & Owonubi, S. J. Biomaterials in tissue engineering. In Tissue Engineering. Applications and Advancements (eds Kesharwani, R. K., Keservani, R. K. & Sharma, A. K.) (Apple Academic Press, 2022). https://doi.org/10.1201/9781003180531.
Guan, F. et al. Characterization of GafchromicTM EBT4 film with clinical kV/MV photons and MeV electrons. Prec. Radiat. Oncol. 7(2), 84–91. https://doi.org/10.1002/pro6.1204 (2023).
Misiarz, A. et al. Design and performance validation of a novel 3d printed thin-walled and transparent electron beam applicators for intraoperative radiation therapy with beam energy up to 12 MeV. Rep. Pract. Oncol. Radiother. 29(3), 329–339. https://doi.org/10.5603/rpor.101092 (2024).
Geant4 – A simulation toolkit. https://geant4.web.cern.ch/ Accessed 02 Jan 2024.
Low, D. A., Harms, W. B., Mutic, S. & Purdy, J. A. A technique for the quantitative evaluation of dose distributions. Med. Phys. 25, 656–661. https://doi.org/10.1118/1.598248 (1998).
Kruszyna-Mochalska, M. et al. Zalecenia Polskiego Towarzystwa Fizyki Medycznej dotyczące kontroli jakości w radioterapii śródoperacyjnej promieniowaniem elektronowym (IOERT) za pomocą mobilnych akceleratorów. Inż. Fiz. Med. 8(1), 7–25 (2019). In Polish.
Istituto Superiori di Sanità (eds Rosi, A. & Viti, V.). Guidelines for quality assurance in intra-operative radiation therapy. Oncología 27(7). https://doi.org/10.4321/S0378-48352004000700013 (2004)
Wołowiec, P. & Kukołowicz, P. F. The analysis of the measurement uncertainty with application of small detectors made of Gafchromic EBT films for the range of doses typical for in vivo dosimetry in teleradiotherapy. Radiat. Meas. 92, 72–79. https://doi.org/10.1016/j.radmeas.2016.08.001 (2016).
Akdeniz, Y. Comparative analysis of dosimetric uncertainty using Gafchromic™ EBT4 and EBT3 films in radiochromic film dosimetry. Radiat. Phys. Chem. 220, 111723. https://doi.org/10.1016/j.radphyschem.2024.111723 (2024).
Miften, M. et al. Tolerance limits and methodologies for IMRT measurement-based verification QA: Recommendations of AAPM task group 218. Med. Phys. 45(4), e53–e83. https://doi.org/10.1002/mp.12810 (2018).
Costa, F., Sarmento, S. & Sousa, O. Assessment of clinically relevant dose distributions in pelvic IOERT using Gafchromic EBT3 films. Phys. Med. 31(7), 692–701. https://doi.org/10.1016/j.ejmp.2015.05.013 (2015).
Severgnini, M., de Denaro, M., Bortul, M., Vidali, C. & Beorchia, A. In vivo dosimetry and shielding disk alignment verification by EBT3 GAFCHROMIC film in breast IOERT treatment. J. Appl. Clin. Med. Phys. 16(1), 112–120. https://doi.org/10.1120/jacmp.v16i1.5065 (2014).
Fastner, G. et al. ESTRO IORT task force/acrop recommendations for intraoperative radiation therapy with electrons (IOERT) in breast cancer. Radiother. Oncol. 149, 150–157. https://doi.org/10.1016/j.radonc.2020.04.059 (2020).
Moghaddam, S. H. Z., Baghani, H. R. & Mahdavi, S. R. Construction and performance evaluation of a buildup bolus for breast intraoperative electron radiotherapy. Radiat. Phys. Chem. 174, 108952. https://doi.org/10.1016/j.radphyschem.2020.108952 (2020).
van Kampen, M. et al. Correlation of intraoperatively irradiated volume and fibrosis in patients with soft-tissue sarcoma of the extremities. Int. J. Radiat. Oncol. Biol. Phys. 51(1), 94–99. https://doi.org/10.1016/s0360-3016(01)01620-0 (2001).
Falco, M. et al. Analysis of breast cosmetic effects 3 years after breast-conserving surgery and intraoperative radiotherapy with and without adjuvant whole breast irradiation. Breast J. 26(5), 882–887. https://doi.org/10.1111/tbj.13767 (2020).
Ciabattoni, A. et al. Intra-operative electron radiation therapy (IOERT) anticipated boost in breast cancer treatment: An Italian multicenter experience. Cancers 14(2), 292. https://doi.org/10.3390/cancers14020292 (2022).
Mihailescu, D. & Botezatu, D. Internal shielding and electron backscattering factors for IOERT beams. Rom. J. Phys. 67, 702 (2022).
Catalano, M., Agosteo, S., Moretti, R. & Andreoli, S. Montecarlo simulation code in optimisation of the intraoperative radiation therapy treatment with mobile dedicated accelerator. In J. Phys: Conf. Ser. Vol. 74, 021002. https://doi.org/10.1088/1742-6596/74/1/021002 (2007).
Author information
Authors and Affiliations
Contributions
A.R. — the concept of the study, literature analysis, Monte Carlo simulations, manuscript writing, phantom design, and data analysis; M.K.-M. — EBT4 measurements, data export and writing the manuscript; B.P. — EBT4 measurements, literature analysis; G.B. — phantom design and fabrication; A.J. — literature analysis, writing the manuscript; P.A. — the concept of the study, supervision of Monte Carlo simulation, data management and analysis, literature analysis, and writing the manuscript; T.P.— the concept of the study, supervision of Monte Carlo simulation, data management and analysis, literature analysis, and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ryczkowski, A., Kruszyna-Mochalska, M., Pawałowski, B. et al. Shielding disc backscatter calculations in intraoperative radiotherapy using a Monte Carlo simulation based on the method of energy spectra reconstruction. Sci Rep 15, 12431 (2025). https://doi.org/10.1038/s41598-025-97522-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97522-1