Abstract
HER2-positive metastatic breast cancer (MBC) represents a challenging subtype of breast cancer, characterized by aggressive disease and poor clinical outcomes. Trastuzumab emtansine (TDM1), an antibody–drug conjugate combining trastuzumab and emtansine, has demonstrated efficacy in clinical trials as a second-line treatment for patients progressing after prior therapies. This study aims to provide real-world evidence on the efficacy and safety of TDM1 in HER2-positive MBC patients. A retrospective analysis was conducted on 70 HER2-positive MBC patients treated with TDM1 at our centre between January 2020 and December 2022. Clinical characteristics, progression-free survival (PFS), overall survival (OS), response rates, and toxicity were evaluated using hospital records. PFS and OS were calculated using Kaplan–Meier methods, and survival curves were compared with log-rank tests. The median age of patients was 47 years, with a majority presenting with advanced disease and prior treatment lines. The median PFS was 6.1 months (95% CI, 4.5–7.6), and the median OS was 14.4 months (95% CI, 10.2–18.0). The objective response rate was 75.7%, with 12.8% achieving a complete response and 62.8% a partial response. PFS was significantly longer in hormone receptor-positive patients compared to hormone receptor-negative patients (8.1 vs. 4.1 months, p = 0.035). Toxicity was manageable, with grade 3–4 adverse events including elevated transaminases (8.5%), thrombocytopenia (5.7%), and anemia (4.2%). The efficacy of TDM1 in this real-world cohort aligns with clinical trial data, though PFS and OS were somewhat lower compared to trials, likely due to the inclusion of patients with more extensive disease and prior treatments. Notably, TDM1 demonstrated activity against CNS metastases and a manageable safety profile, with higher incidence of hepatic and hematologic toxicities. Our study supports the use of TDM1 as a viable option for treating HER2-positive MBC in routine clinical practice, confirming its effectiveness and safety profile observed in clinical trials.
Similar content being viewed by others
Introduction
Breast cancer is the most diagnosed cancer type, accounting for 1 in 8 cancer diagnoses worldwide. In 2020, there were about 2.3 million new cases of breast cancer globally and about 685,000 deaths from it. Breast cancer incidence rates are highest in countries that have undergone economic transition1,2. Over the past decade, a novel molecular classification of breast cancer has emerged, featuring the human epidermal growth factor receptor 2 (HER2), a component of the ErbB family. Amplification of HER2 occurs in approximately 20% of invasive breast cancers and correlates with aggressive disease and unfavourable clinical outcomes3,4. Treatment strategies for HER2-positive breast cancer have rapidly evolved following the identification of HER2 as a prime target for anticancer therapies. The combination of trastuzumab, pertuzumab, and a taxane has significantly prolonged progression-free survival (PFS) in randomized trials and now represents the standard first-line therapy for HER2-positive breast cancer5. However, disease relapse eventually necessitates second-line treatment. Multiple studies have demonstrated the efficacy of trastuzumab plus emtansine (TDM1) in HER2-positive breast cancer patients who have progressed after prior therapy6,7,8,9.
TDM1 is an antibody drug conjugate that merges the functions of trastuzumab and emtansine. Trastuzumab, a humanized monoclonal antibody, selectively binds to the HER2 membrane receptor, inducing apoptosis and activating cellular immunity. Emtansine, an antimicrotubule agent, inhibits tubulin polymerization and exhibits potency approximately 40 times greater than taxanes in in vitro studies, while maintaining efficacy without increasing toxicity10. In TDM1, trastuzumab and emtansine are linked by a stable thioether linker, ensuring the conjugate’s stability until it reaches the HER2 receptor target. Upon receptor-mediated internalization, emtansine is released into the cytoplasm, where it disrupts microtubules and inhibits HER2 signaling, leading to cell-cycle arrest and apoptosis11,12. TDM1 have shown significant clinical benefit in EMILIA and TH3RESA study8,13,14 Though Trastuzumab deruxtecan has now become available, and is preferred drug in this setting, the cost of the treatment precludes its utilization in a majority of the patients in resource-constrained settings. Thus, TDM1 remains the most common treatment for HER2-positive breast cancer patients who experience disease progression during or within 12 months after adjuvant treatment with trastuzumab, as well as for those who relapse after initially responding to treatment with trastuzumab plus a taxane, with or without pertuzumab.
While clinical trials provide crucial evidence of treatment efficacy, they may not fully capture the complexities of routine clinical care. Real-world data studies address these gaps and offer long-term efficacy insights to complement trial findings. In our retrospective real-world study, we assessed the efficacy of TDM1 in a cohort of HER2-positive breast cancer patients treated at our center15.
Patient and methods
This study is a single-centre, retrospective analysis conducted at our hospital, focusing on the efficacy and safety of trastuzumab emtansine (TDM1) in HER2-positive metastatic breast cancer (MBC) patients.
Patient selection
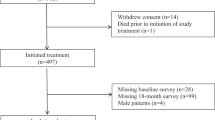
We reviewed the medical records of patients treated with T-DM1 between January 2020 and December 2022. Inclusion criteria were: (1) Confirmed HER2-positive breast cancer, proven by biopsy, Her2 was performed by standard institutional protocol as summarised in a subsequent paragraph (2) metastatic disease confirmed by imaging and (3) receipt of at least one cycle of TDM1 treatment during the study period. Patients with incomplete records or those who did not receive at least one cycle of TDM1 were excluded.
HER2 testing methodology
HER2 testing was performed using two primary methods: immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). These diagnostic assays were integral to confirming HER2 positivity in our study population.
Immunohistochemistry (IHC)
IHC was conducted on formalin-fixed, paraffin-embedded breast cancer tissue samples. A standard IHC procedure involved deparaffinization, rehydration, and antigen retrieval followed by incubation with a HER2-specific monoclonal antibody. The presence of HER2 protein overexpression was assessed by evaluating the intensity and pattern of staining under a light microscope. HER2 expression was categorized into four levels: 0 (no staining), 1 + (weak, incomplete membrane staining), 2 + (moderate, complete membrane staining), and 3 + (strong, complete membrane staining). A score of 3 + was considered positive, indicating high HER2 overexpression. In cases where the IHC score was 2 +, further testing with FISH was employed to confirm HER2 amplification.
Fluorescence in situ hybridization (FISH)
FISH analysis was used to detect HER2 gene amplification in cases with ambiguous IHC results (2 +). This technique involved applying fluorescently labeled DNA probes specific for the HER2 gene and the chromosome 17 centromere to tumor cell nuclei. The number of HER2 gene copies was quantified relative to the number of chromosome 17 centromere copies. A HER2/CEP17 ratio greater than 2.0 or HER2 gene copy number exceeding 6.0 signals per cell was indicative of HER2 gene amplification. FISH provided a quantitative assessment, confirming HER2 positivity in patients with equivocal IHC results.
Data collection
Clinical characteristics, treatment details, and outcomes were extracted from electronic health records. Data collected included patient demographics (age, sex, menopausal status), ECOG performance status at the time of T-DM1 initiation, hormone receptor status (estrogen receptor [ER] and progesterone receptor [PR]), number of metastatic sites, presence of visceral and central nervous system (CNS) metastases, and prior treatment history.
Treatment regimen
All patients received TDM1 administered as a single agent, typically at a dose of 3.6 mg/kg every 3 weeks. The treatment duration varied based on clinical response and tolerability.
Outcome measures
Progression-free survival (PFS)
Defined as the time from the start of T-DM1 treatment to disease progression or death from any cause. Disease progression was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, based on imaging studies and clinical evaluations.
Overall survival (OS)
Defined as the time from the start of T-DM1 treatment to death from any cause. Patients alive at the last follow-up were censored.
Response evaluation
Contrast CT/PET scans were performed for response evaluation as per the standard institutional practice. MRI of brain was performed in patients who had symptoms related to CNS metastasis. Responses to TDM1 were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. Objective response rate (ORR) was calculated as the proportion of patients achieving CR or PR.
Toxicity assessment
Adverse events were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. We recorded any grade 3 or 4 toxicities and documented their incidence.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Kaplan–Meier survival curves were constructed to estimate median PFS and OS, with 95% confidence intervals (CIs) reported. PFS and OS were compared between different patient subgroups using the log-rank test. The significance level was set at p ≤ 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Armonk, NY), version 24.
Subgroup analysis
Subgroup analyses were conducted based on hormone receptor status, presence of visceral and CNS metastases, and number of metastatic sites. We compared PFS and OS among these subgroups to identify factors influencing treatment outcomes.
Ethical considerations
This study adhered to ethical standards in line with the Declaration of Helsinki. Patient confidentiality was maintained throughout the study. Written informed consent was not required due to the retrospective nature of the study, but patient data were anonymized to ensure privacy. The study received approval from the hospital’s ethics committee.
Results
Baseline characteristics of the patients
A total of 70 Her2 positive MBC patients who satisfied the inclusion and exclusion criteria were included in this retrospective study. The baseline characteristics of the patients is presented in Table 1. The median age was 47 years, with 46.3% being pre-menopausal. At the time of therapy, 7.1% of patients had an ECOG status of 0, 85.7% had a status of 1, and 7.1% had a status of 2. Additionally, 64.3% of patients were triple-positive. Of these patients, 54.3% were diagnosed with de novo metastatic disease, while the remaining 45.7% initially presented with local or locally advanced disease before developing metastasis. Visceral disease involvement was observed in 65.7% of patients, with 40% experiencing central nervous system (CNS) metastases. The median number of previous therapy lines was 2.5, with all patients having previously received trastuzumab, either as adjuvant treatment or for metastatic disease. Other treatments included capecitabine with lapatinib (70% of patients), anthracyclines (40%), taxanes (78.6%), hormonal therapy (57%), gemcitabine and carboplatin (18.5%), and Vinorelbine (2.8%). Most patients (66.6%) received TDM1 as the second or third line of treatment for metastatic disease, with a median of 6 cycles administered (range: 1–38).
Clinical outcomes
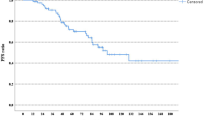
All 70 patients were evaluated for PFS, OS, and response. Median PFS was 6.1 months (95% CI, 4.5- 7.6) (Fig. 1A). Median OS was 14.4 months (95% CI, 10.2–18.0) (Fig. 1B). The objective response rate was 75.7%. Nine patients (12.8%) achieved a complete response, 44 (62.8%) a partial response, 12 (17.1%) had stable disease as their best response. Five (7.1%) patients progressed on their first reassessment scan.
Among the twenty-eight patients with CNS metastases, PFS was 6.7 months (95% CI, 0.2–13.1), compared to 6.1 months (95% CI, 4.7–7.4) for the 42 patients without CNS metastases (p = 0.33). OS was 13.6 months (95% CI, 11.8–15.3), while 17.5 months (95% CI, 11.8–23.3) for patient without CNS metastasis) (p = 0.35).
Among the 45 HR + patients, PFS was 8.1 months (95% CI, 4.5–11.8) when compared to 4.1 months (95% CI, 2.3- 5.9) for HR Neg patients (p = 0.035). The OS for HR + patients was 16.7 (95% CI, 12.4–20.9), while for HR neg patients, it was 13.1 months (95% CI, 10.2- 16.1) (p = 0.2) Among the 46 patients with visceral metastasis, PFS was 5.1 months (95% CI, 3.3.−6.9) when compared to 6.7 months (95% CI, 3.0- 10.3) (p = 0.33) for patients without visceral disease. OS for patients with visceral disease was 13.1 (95% CI, 10.2 −16.9) vs. 26.0 months (95% CI, 16.2–35.7) (p = 0.012) for patients without visceral metastasis (Fig. 1C). Among the 31 patients with 3 metastasis, PFS was 7.4 months (95% CI, 3.5.−11.4) when compared to 4.4 months (95% CI, 2.4–6.4) (p = 0.009) for patients with more than 3 metastases. OS for patients with 3 or less metastasis was 17.8 months (95% CI, 16.1–19.5) when compared to 13.6 months (95% CI, 8.6–18.6) (p = 0.056) for patients having > 3 metastases. According to different lines of treatment, PFS for second line and third line or beyond was 6.4 months and 5.5 months, respectively (p = 0.51). The corresponding OS was 14 months and 17.8 months, respectively (p = 0.84).
Toxicities
The main grade 3–4 toxicities were elevated transaminases (8.5%), thrombocytopenia (5.7%), anemia (4.2%), and neutropenia (1.4%). Table 2 summaries all the toxicities of the cohort.
Discussion
Based on the results of two clinical trials, EMILIA and TH3RESA, TDM1 is utilised as a standard of treatment for HER2-positive breast cancer, both as second- or further-line treatment for advanced or metastatic disease and as first-line treatment in the case of early progression to adjuvant treatment6,7,8,9,13. It should be highlighted that trastuzumab deruxtecan has now become available and has shown improved treatment outcomes as compared to TDM1 based on DB03 results16. However, the cost issues preclude its widespread use in most of the patients and TDM1 remains the most commonly utilised antibody–drug conjugate in this setting. This study is a retrospective analysis of patients treated at a single centre and offers real-world evidence to reinforce and uphold the conclusions drawn from the two significant clinical trials (Table 3).
The median age of our patients was 47 years (ranging from 29 to 68), compared to 53 in the EMILIA study, while 83% of patients in the TH3RESA study were under the age of 65. In our study, the PFS was 6.1 months, which closely resembles the 6.2 months reported in the TH3RESA study but falls short of the 9.6 months observed in the EMILIA study, which included patients who had undergone fewer prior treatment lines. OS in our patients was 14.1 months which is lesser than that attained by the patients in the EMILIA study (30.9 months) and the TH3RESA study (22.7 months). To add, PFS was longer than 12 months in seven of our patients, including two patients who were progression-free for more than 27 months. All these patients did not exhibit visceral metastatic disease. Literature has also documented instances of remarkable and prolonged responses to TDM1, particularly among patients lacking visceral disease17. Furthermore, 55.7% of our patients had metastases in three or more sites, contrasting with 37% in the EMILIA study and 64% in the TH3RESA study. Unlike the clinical trials, our study encompassed all patients who could potentially derive benefit from TDM1. The inclusion of these patients with a poorer prognosis, may be the cause for lesser OS in our study. Besides, 40% of patients in our study had CNS metastases, compared to just 9% and 10% in the EMILIA and TH3RESA trials, respectively. We observed a statistically significant difference in OS in patients with and without brain metastases, which indicated that TDM1 exhibited activity against these metastases as well18,19.
In our study, 64.3% were triple-positive, in contrast to 55% in the EMILIA study and 52% in the TH3RESA study. PFS was significantly better in HR + patients (median 8.1 months vs 4.1, p = 0.035), although there was no significant difference in OS. One possible explanation is that these patients might have already experienced prolonged survival with previous lines of treatment, enabling them to progress to TDM1 in more advanced treatment stages. This could potentially introduce a bias into the selection of our patients, which would not have been present in the clinical trials. Consequently, HER2-positive but hormone receptor-negative patients with a poorer prognosis due to aggressive disease behaviour may have been excluded from consideration. This selection process could have introduced a negative bias. Despite the generally lower rates of pathological complete response to neoadjuvant treatment observed in triple-positive breast cancer patients, our findings suggest that this initial response does not necessarily translate to poorer outcomes with TDM1 in the long term20,21,22.
In our study, we observed a higher incidence of elevated transaminases and neutropenia compared to the EMILIA and TH3RESA studies. A safety analysis across six studies of T-DM1 in HER2-positive breast cancer, involving 884 patients, identified asthenia as the most common adverse effect, followed by nausea and vomiting23. Thrombocytopenia was reported in 32% of patients, while 23% experienced elevated transaminases. Overall, our patients exhibited higher rates of hepatotoxicity and hematological toxicities, although most were of grade 1–2 severity. These elevated rates of grade 1–2 toxicity in our study may be attributed to less stringent dosing control of TDM1 compared to clinical trials or to a higher number of TDM1 cycles administered. Despite our series having a median of six cycles of TDM1, which is lower than the 10.1 and 10.5 cycles observed in the EMILIA and TH3RESA studies, TDM1 demonstrated efficacy in treating HER2-positive metastatic breast cancer with an excellent safety and tolerability profile.
Conclusions
Our real-world data corroborate and endorse the findings of the two major phase III trials, highlighting the utility of TDM1 in routine clinical practice for treatment of metastatic Her2 positive breast cancer patients.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Heer, E. et al. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 8, e1027–e1037 (2020).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36(20), 2105–2122 (2018).
Ross, J. S. et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 14(4), 320–368 (2009).
Baselga, J. et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366(2), 109–119 (2012).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367(19), 1783–1791 (2012).
Krop, I. E. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann. Oncol. 26(1), 113–119 (2015).
Krop, I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomized, open-label, phase 3 trial. Lancet Oncol. 15(7), 689–699 (2014).
Welslau, M. et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer 120(5), 642–651 (2014).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380(7), 617–628 (2019).
Junttila, T. T., Li, G., Parsons, K., Phillips, G. L. & Sliwkowski, M. X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib-insensitive breast cancer. Breast Cancer Res. Treat. 128(2), 347–356 (2011).
Kovtun, Y. V. et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 70(6), 2528–2537 (2010).
Amiri-Kordestani, L. et al. FDA approval: Ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res. 20(17), 4436–4441 (2014).
Krop, I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomized open-label phase 3 trial. Lancet Oncol. 18(6), 743–754 (2017).
Garrison, L. P., Neumann, P. J., Erickson, P., Marshall, D. & Mullins, C. D. Using real-world data for coverage and payment decisions: The ISPOR real-world data task force report. Value Health. 10(5), 326–335 (2007).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401(10371), 105–117 (2023).
Hopkins, A. M., Rowland, A., Logan, J. M. & Sorich, M. J. Primary predictors of survival outcomes for HER2-positive advanced breast cancer patients initiating ado-trastuzumab emtansine. Breast 46, 90–94 (2019).
Bartsch, R. et al. Activity of T-DM1 in HER2-positive breast cancer brain metastases. Clin. Exp. Metastasis. 32(7), 729–737 (2015).
Leone, J. P. & Lin, N. U. Systemic therapy of central nervous system metastases of breast cancer. Curr. Oncol. Rep. 21(5), 49 (2019).
Gianni, L. et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 17(6), 791–800 (2016).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13(1), 25–32 (2012).
Hurvitz, S. et al. Pathologic complete response rates after neoadjuvant trastuzumab emtansine (T-DM1) plus pertuzumab vs. docetaxel plus carboplatin plus trastuzumab plus pertuzumab (TCH+P) treatment in patients with HER2-positive early breast cancer (KRISTINE/TRIO-021). J. Clin. Oncol. 34((15_suppl)), 500 (2016).
Diéras, V. et al. Trastuzumab emtansine in HER2-positive metastatic breast cancer: An integrated safety analysis. J. Clin. Oncol. 32(25), 2750–2757 (2014).
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Contributions
A.K., A.G., and B.S. wrote the main manuscript text and A.P. prepared the results, tables, and Figs. 1–3. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gupta, A., Sansar, B., Mishra, B.K. et al. Real-world data on trastuzumab emtansine (TDM1) efficacy and safety: Results of a single-centre retrospective study of HER2-positive metastatic breast cancer patients. Sci Rep 15, 18669 (2025). https://doi.org/10.1038/s41598-025-97923-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97923-2