Abstract
The incidence of Gastric cancer (GC) has shown a sharp upward trend, and patients with GC complicated by diabetes exhibit significantly worse clinical outcomes and prognosis compared to those without diabetes. Traditional Chinese medicine has played a crucial role in the treatment of both GC and diabetes. Currently, Banzhilian(Scutellaria barbata D. Don) is utilized in the treatment of GC; however, the specific small-molecule monomers it contains and their mechanisms of action have not yet been fully elucidated. This study aims to explore the mechanism of quercetin, a key component of Banzhilian, through network pharmacology, molecular docking, molecular dynamics (MD) simulation, bioinformatics, and in vitro and in vivo experiments. Initially, core targets and key pathways involved in the treatment of diabetes-associated GC (GC-diabetes) were identified using public databases. Subsequently, molecular docking, MD simulation, and survival analysis were performed. Experimental validation included CCK-8 assays, colony formation assays, apoptosis detection, cell cycle analysis, wound healing assays, Transwell migration assays, Western blotting, and mouse subcutaneous tumor formation experiments to evaluate the effects of quercetin, as an active monomer in Banzhilian, on Gastric cancer cells (HGC-27-HG cells) under high-glucose conditions. In this study, quercetin was identified as the key active component, with AKT1, TP53, JUN, MYC, and CCND1 recognized as the target genes, and the PI3K/AKT signaling pathway as the primary regulatory pathway. The results of the study indicate that the proliferation, migration, and invasion capabilities of HGC-27-HG cells are significantly higher than those of HGC-27 cells. However, quercetin inhibited the growth of HGC-27-HG cells, promoted apoptosis, induced cell cycle arrest at the G0/G1 phase, and reduced the cells’ migration and invasion abilities. Furthermore, it downregulated the expression of target genes and their phosphorylation levels. The experimental findings confirmed that quercetin, as an active monomer in Banzhilian, suppresses the proliferation of HGC-27-HG cells by inhibiting the PI3K/AKT/MYC pathway, promoting apoptosis, blocking cell cycle progression, and inhibiting cell migration and invasion.
Similar content being viewed by others
Introduction
The prevalence of Gastric cancer (GC) has shown substantial escalation in recent times, with statistics indicating 970,000 new diagnoses and approximately 660,000 fatalities documented in 2022, ranking fifth globally in both incidence and mortality1. In China, 360,000 novel GC instances emerged in 2022, constituting 7.62% of all new cancer diagnoses, also ranking fifth. Additionally, 260,000 GC-related deaths occurred, making up 10.11% of all cancer deaths and ranking third2. The diagnosis rate of early GC remains low, and most individuals are diagnosed at the middle to late stages of the disease3. Diabetes mellitus is a group of endocrine and metabolic disorders marked by chronic hyperglycemia, resulting from a range of pathogenic factors that impair insulin secretion and action4. Recent investigations have shown a notable connection between diabetes and an increased risk of diverse cancers, particularly endometrial cancer, colorectal cancer, and postmenopausal breast cancer5. In addition, there is mounting evidence linking diabetes with GC. Research on the relationship between diabetes and GC has demonstrated that hyperglycemia, hyperinsulinemia, and insulin resistance are closely associated with the development of GC6. He et al. reported that hyperglycemia triggers miR-26-5p to downregulate PFKFB3 expression, promoting epithelial-mesenchymal transition (EMT) in GC7. Yu et al. found that inhibition of Pin1 and BRD4 markedly reduced the multiplication and motility of high-glucose-induced GC cells, while high glucose promoted tumor development and lung metastasis by activating Pin1 and BRD4 expression8. Some studies have indicated that diabetic patients may face higher risks and lower survival rates during Gastric cancer treatment, and elevated fasting blood glucose levels may also increase the risk of developing Gastric cancer9,10,11. Numerous studies have demonstrated that diabetic Gastric cancer patients experience improved glycemic control and a significant amelioration of insulin resistance following gastrectomy12,13.
On the basis of these findings, diabetes appears to contribute to GC advancement. Despite advances in surgical treatment and chemotherapy, the overall survival rate (OS) for individuals with GC remains low due to challenges such as tumor metastasis, recurrence, and drug resistance14. The study by Zhao et al. revealed that hyperglycemic conditions enhance the expression of Nampt and Sirt1, which subsequently increases the expression of mutant p53. This leads to the upregulation of P-gp and downregulation of Topo-IIα, thereby promoting the proliferation of Gastric cancer cells and reducing their sensitivity to chemotherapeutic agents15.Studies have shown that metformin, a commonly used hypoglycemic agent, also possesses significant anti-tumor potential16. Co-treatment with metformin and 5-FU can overcome chemoresistance in Gastric cancer cells by downregulating the expression levels of WNT5A, MDR1, P-gp, and CD4417. However, excessive use of metformin may lead to smaller fetal sizes during pregnancy18. Given the toxic and side effects of Western medicines, the use of natural plants and their bioactive compounds in the treatment of GC-diabetes is likely to become a major therapeutic direction in the future.
Numerous investigations have demonstrated that traditional Chinese medicine (TCM) can prevent and treat GC. Zhang et al. showed that berberine suppressed autophagy by blocking MAPK/mTOR/p70S6K and Akt pathways, consequently diminishing human GC cell proliferation in both in vitro and in vivo19. Yu et al. isolated a cold-water soluble polysaccharide (APS4) from Astragalus, which induced significant apoptosis in MGC-803 cells by inhibiting the S-phase of the cell cycle and activating the intrinsic mitochondrial apoptotic pathway20. According to Fu et al., the combination of Coptis and dried ginger inhibited tumorigenesis by modulating glucose metabolism via the LDHA and SLC2A1 genes21. Scutellaria barbata D. Don, known as Ban Zhi Lian in Chinese, is a widely used herbal medicine in China, commonly found in moist environments such as hillsides, grasslands, roadsides, and fields. It contains a variety of bioactive components, primarily including flavonoids, phenolic acids, volatile oils, and polysaccharides. Its main therapeutic effects encompass clearing heat and detoxifying, promoting blood circulation and resolving stasis, reducing swelling and alleviating pain, as well as antitumor, anti-inflammatory, immunomodulatory, and hepatoprotective properties. It exhibits a range of pharmacological activities, including antioxidant, anti-inflammatory, and anticancer characteristics22, Extensive research has confirmed its preventive effects against various cancers. Xue et al. reported that Banzhilian suppressed the growth and metastasis of cervical cancer cells and triggered apoptosis via the miR-195-5p/LOXL2 pathway23. Liu et al. found that the flavonoids in Banzhilian exert anti-tumor activity in colorectal cancer by inhibiting autophagy and promoting apoptosis through the ATF4/sestrin2 pathway24. Sheng et al. demonstrated that Banzhilian markedly diminished cell viability and clonogenic growth in a dose-dependent manner, while also inducing apoptosis and G2/M cell cycle arrest via the inactivation of the PI3K/AKT signaling pathway25. In Gastric cancer, according to the research by Jin et al., the crude extract of Banzhilian can inhibit the proliferation of human gastric adenocarcinoma cells and promote apoptosis by regulating caspase, MAPK, and ROS-dependent pathways26. Meanwhile, studies have also shown that polysaccharides extracted from Scutellaria barbata can inhibit high glucose-induced proliferation, migration, and angiogenesis of HRVECs by blocking the activation of the MEK/ERK pathway and the VEGF/VE-cadherin axis27. However, the therapeutic effects of Banzhilian on GC-diabetes have yet to be studied, and its underlying mechanisms remain unclear.
To address this gap, this investigation employed network pharmacology (NP), molecular docking, molecular dynamics (MD) simulation, and bioinformatics analysis to identify Banzhilian’s bioactive constituents and therapeutic targets. The molecular mechanisms underlying the anti-diabetic Gastric cancer effects of quercetin were explored, followed by experimental validation, providing robust data support for the potential of quercetin in treating GC-diabetes. (Fig. 1).
Methods
Acquisition of the target gene of Banzhilian on GC-diabetics
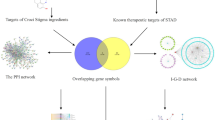
Disease genes associated with both diabetes and GC were identified using several online databases, including GeneCards (https://www.genecards.org/), OMIM (https://omim.org/), DrugBank (https://go.drugbank.com/about)28, PharmGKB (http://lilab-ecust.cn/pharmmapper/index.html)29, and TTD (https://db.idrblab.net/ttd/)30. The overlapping genes between diabetes and GC were selected, and these overlapping genes were considered co-pathogenic genes for GC-diabetes. The active components and target sites of Banzhilian were ascertained utilizing the TCMSP database (https://old.tcmsp-e.com/tcmsp.php)31, and the corresponding gene symbols were standardized. The target genes of Banzhilian were then intersected with the co-pathogenic genes of GC-diabetes to identify the communicative genes, which were considered the prospective targets of Banzhilian in GC-diabetes treatment.
Construction of the PPI network
To develop the protein-protein interaction (PPI) network of the target genes, Banzhilian’s target genes were imported into the STRING database(https://string-db.org/)30, and the network was processed utilizing the CytoNCA plug-in. The essential targets of Banzhilian in treating GC-diabetes were then ascertained.
Enrichment analysis
Further, GO and KEGG enrichment analyses were executed utilizing the Metascape database (https://metascape.org/)33 to understand the biological processes and pathways implicated in Banzhilian’s action on GC-diabetes34,35,36.
Survival analysis
To evaluate the clinical significance of the key target genes, survival analysis was executed utilizing the Kaplan-Meier Plotter database (http://kmplot.com), an online survival analysis platform that integrates gene expression data from multiple sources and is one of the most comprehensive and authoritative survival analysis tools available. This examination was employed to evaluate the link between the expression of key genes and the survival rates of individuals with GC.
Molecular Docking
For molecular docking, the structure of the key active compound quercetin was procured from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and its 3D structure was generated utilizing Chem3D software, saved in “.mol2” format. The structural data of the core target gene was retrieved from the RCSB PDB database (https://www.rcsb.org/) and imported into Open-Source PyMOL(Schrodinger, LLC.), where water molecules and residual ligands were eliminated. The protein structure was subsequently hydrogenated utilizing AutoDockTools (v1.5.6, http://autodock.scripps.edu/) and saved in “.pdbqt” format. Lastly, the interaction potential between quercetin and the essential target proteins was evaluated through molecular docking simulations performed with Autodock Vina(v1.1.2, http://vina.scripps.edu/). The molecular docking interactions were visualized and analyzed using Open-Source PyMOL for 3D representation and LigPlot+(v2.2.) for 2D interaction diagrams37.
MD simulation
Based on the molecular docking outcomes, the protein-ligand complexes exhibiting the highest absolute binding free energies were selected for MD simulations. The investigations were conducted utilizing the GROMACS 2023.3 software package. The amber14SB force field was utilized for the protein, while the gaff force field was applied for small molecule ligands. The ligand’s topology files were constructed using the ACPYPE software38,39. The atomic charges for the ligands were calculated using the Restrained Electrostatics Potential (RESP) method, with charges computed at the B3LYP/6-311G** level using ORCA software and Mutifwn software40. The TIP3P water model was utilized to solvate the protein-ligand complex. To maintain electrical neutrality, NA + and CL- ions were added to the simulation box. The initial structure was subsequently optimized for energy using the conjugate gradient protocol, with the minimization process terminating once the highest force value fell beneath 10 kJ/mol. Following energy minimization, the setup underwent thermal adjustment for 100 ps at 300 K within an NVT ensemble utilizing periodic boundary parameters. The simulation employed a 2 fs timestep, with hydrogen-containing covalent bonds constrained through LINCS protocol41. A V-rescale thermostat maintained the 300 K temperature, while PME methodology handled electrostatic forces with a 1.4 nm cutoff, matching the van der Waals interaction limit. The process included a brief 100 ps equilibration phase, succeeded by main simulations utilizing 2 fs steps in an NPT ensemble under identical protocols. The Parrinello-Rahman algorithm regulated pressure at 1.0 atm. The final simulation run was conducted for 100 ns at 300 K and 1 atm for all the simulation systems. The resulting MD simulation trajectories were analyzed to observe key metrics such as hydrogen bonding, the radius of gyration, Root Mean Square Fluctuation (RMSF), and Root Mean Square Deviation (RMSD) using GROMACS functions (gmx_hbound, gmx_gyrate, gmx_rms, and gmx_rmsd). The trajectories were visualized using VMD software (version 1.9.3)42.
Cell culture
The human Gastric cancer HGC-27 cells (CAT: PWE-HU064) were purchased from Dalian Meilun Biotechnology Co., Ltd. These cells were cultured under standard conditions in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution. To simulate a high-glucose environment, a high-glucose medium was prepared by adding 200 g/L D-glucose to sugar-free RPMI-1640 medium containing 10% FBS and 1% penicillin-streptomycin solution, resulting in a final glucose concentration of 25 mmol/L. This medium was used to culture high-glucose Gastric cancer cells, referred to as HGC-27-HG. For drug treatment, the cells were incubated in RPMI-1640 medium containing 200 g/L D-glucose but without FBS or penicillin-streptomycin solution to dilute the drugs. All cells were maintained in a humidified incubator at 37 °C with 5% CO2. The cells used in this experiment were adherent cells. After 24 h of culture, when the cell density reached approximately 90%, the cells were digested using trypsin and evenly transferred to the same type of culture flasks or seeded into multi-well plates for subsequent experimental procedures.
Experimental reagents and instrument
Reagents used in the study included fetal bovine serum (Gibco, USA), Trypsin-EDTA (Gibco, USA), RPMI-1640 (Pricella Biotechnology Co., Ltd., Wuhan, China), sugar-free RPMI-1640 (Pricella Biotechnology Co., Ltd., Wuhan, China), 1% penicillin-streptomycin (Sigma, Poland), D-glucose solution (200 g/L) (Pricella Biotechnology Co., Ltd., Wuhan, China), quercetin (Sigma, USA), Cell Counting Kit-8 (Sigma, USA), Cell Apoptosis Detection Kit (KeyGEN Bio TECH Corp., Ltd., Jiangsu, China), Matrigel (Corning, USA), EdU Cell Proliferation Kit with Alexa Fluor 488 (Beyotime Biotechnology, Shanghai, China), Calcein/PI Cell Viability/Cytotoxicity Assay Kit (Beyotime Biotechnology, Shanghai, China), and antibodies for GAPDH, β-actin, E-cadherin, Vimentin, N-cadherin, Snail, PI3K, p-PI3K, Akt, p-Akt, and Myc (Abcam, USA), Inverted microscope(Olympus, Japan, IX70-S8F2).
Determination of IC50
A total of 100 µL of HGC-27-HG cell suspension at a concentration of 20,000 cells/mL was added to each well of a 96-well plate and incubated until the cells adhered to the surface. Quercetin was added at concentrations of 1.56, 3.13, 6.25, 12.5, 25, 50, 100, and 200 µmol/L, and cells were kept for 24 h. After treatment, 10 µL of CCK-8 reagent was introduced to each well. Following a 2-h incubation at 37 °C, absorbance was ascertained at 450 nm utilizing an enzyme reader.
Cell viability assay
A total of 100 µL of HGC-27-HG cell suspension at a concentration of 20,000 cells/mL was added to each well of a 96-well plate and incubated until the cells adhered to the surface. Quercetin was added at concentrations of 20, 60, and 100 µmol/L. The cells underwent incubation for 24, 48, and 72 h, with CCK-8 reagent introduced to each well 2 h prior to measuring absorbance at 450 nm.
Clonal formation experiment
For the colony formation assay, HGC-27 and HGC-27-HG cells were evenly seeded into 6-well plates. After cell adherence, the medium was replaced with drug-containing medium, and the cells were incubated for 24 h. Subsequently, the cells were digested and re-seeded into new 6-well plates at a density of approximately 500 cells per well. Following 1–2 weeks of incubation, the cell colonies were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The colonies were then observed and analyzed under a microscope.
EdU staining
HGC-27 and HGC-27-HG cells were collected and seeded into 6-well plates. After cell adherence, the medium was replaced with drug-containing medium, and the cells were incubated for 24 h. Subsequently, the EdU working solution was added, and the cells were further incubated for 2 h. Following incubation, the medium was discarded, and 1 ml of 4% paraformaldehyde was added to each well to fix the cells for 15 min. After removing the fixative, the samples were washed three times with 1 ml of PBS washing buffer containing 3% BSA, each time for 5 min. Subsequently, 1 ml of PBS permeabilization buffer containing 0.3% Triton X-100 was added to each well and incubated for 15 min. After removing the permeabilization buffer, the samples were washed 1–2 times with 1 ml of washing buffer, each time for 3–5 min. After discarding the wash buffer, click reaction mixture (0.5 ml) was introduced to each well, succeeded by light-protected incubation for 30 min at ambient temperature. The reaction mixture was then extracted, and specimens underwent triple washing with buffer for 3–5 min. Following buffer removal, Hoechst33342 solution (1 ml) was introduced to each well, with light-protected incubation for 10 min at ambient temperature. After aspirating the Hoechst33342 solution, specimens underwent triple washing with buffer for 3–5 min. Fluorescence detection was then performed, with Hoechst33342 emitting blue fluorescence and EdU emitting green fluorescence.
Calcein-AM/PI live dead staining
HGC-27 and HGC-27-HG cells were collected and seeded into 6-well plates. After cell adherence, the medium was replaced with drug-containing medium. After 24 h, the old medium was aspirated, and the cells were washed twice with PBS. Subsequently, 1 ml of Calcein-AM/PI detection mixture was added to each well, and the cells were incubated at 37 °C in the dark for 30 min. Following incubation, staining was examined utilizing a fluorescence microscope, with CalceinAM emitting green fluorescence and PI emitting red fluorescence.
Apoptosis detection
HGC-27 and HGC-27-HG cells were collected and seeded into 6-well plates. After cell adherence, the medium was replaced with drug-containing medium, and the cells were incubated for 24 h. Subsequently, the cells were harvested using trypsin digestion solution without EDTA. The cells were rinsed and pelleted. Next, 5 µL of Annexin V-FITC and 5 µL of Propidium Iodide (PI) were introduced, and the cells were kept in the dark at room temperature for 10 min. Flow cytometry analysis was conducted within 1 h. The experimental results were analyzed using FlowJo (v10.8.1).
Cell cycle detection
HGC-27 and HGC-27-HG cells were placed into 6-well plates and exposed to quercetin for 24 h upon reaching roughly 70% confluence. The cells were collected, rinsed, and stabilized utilizing 500 µL of 70% cold ethanol overnight at 4 °C. The fixation solution was discarded, and the cells were centrifuged at 1000 rpm for 3 min. RNaseA/PI working solution (500 µL) was introduced, and the cells were kept for 30 min. Red fluorescence was examined at 488 nm. The experimental results were analyzed using FlowJo (v10.8.1).
Wound scratch detection
HGC-27 and HGC-27-HG cells were collected and seeded in 6-well plates. Once the cell density approached 90%, vertical scratches were made using a pipette tip. The existing medium was substituted with a serum-free medium comprising varying levels of quercetin. Images were procured at the identical observation points at 0 h and 24 h, and the scratch healing rate was computed.
Transwell chamber experiment
For the cell migration assay, HGC-27 and HGC-27-HG cells were seeded in 6-well plates and treated with drugs after cell adhesion. Following 24 h, the cells were collected, resuspended in a serum-free medium, counted, and adjusted to 2.5 × 105 cells/ml. A volume of 200 µL cell suspension was introduced into the upper compartment, while 500 µL serum-supplemented medium was deposited in the lower compartment. After 24 h of incubation, the cells in the upper chamber were removed, fixed with 4% paraformaldehyde for 30 min, and stained with crystal violet for 15 min. Cells adhering to the upper interior surface were cleared away, and images were captured of the remaining cells. For examining invasion, the upper compartment received an even coating of diluted Matrigel, with surplus fluid extracted after a 3-h incubation at 37 °C. The following procedures matched those of the migration protocol.
Immunohistochemical assay
Clinically collected postoperative tissue samples from Gastric cancer patients with concurrent diabetes (fasting blood glucose level: 8.5 mmol/L; postprandial 2-h blood glucose level: 13.3 mmol/L) were preserved in 4% paraformaldehyde for 2 days, subsequently embedded in paraffin blocks, and sectioned to a thickness of 4 μm. The continuous tissue sections were deparaffinized with xylene, rehydrated, and subjected to antigen retrieval by microwave heating in EDTA buffer for 5–8 min, followed by cooling to room temperature. To prevent reagent leakage, the sections were circled with an immunohistochemical pen. Endogenous peroxidase blocking solution was added within the circles and incubated at room temperature for 10 min. Subsequently, blocking serum was applied and incubated at 37 °C for 30 min, after which excess serum was removed. After blocking, the sections were incubated overnight at 4 °C with primary antibodies against AKT1, TP53, MYC, JUN, and CCND1, followed by incubation with secondary antibodies at room temperature for 2 h. Finally, the sections were developed using DAB solution, counterstained with hematoxylin, and evaluated under a microscope. The processing of volunteer tissue samples has been approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical University (BYEFY(2022) Ethics Review No. 29 A) and strictly adheres to the ethical standards of the Declaration of Helsinki. Informed consent was obtained from all participants and/or their legal guardians, and written informed consent forms were signed.
Western blot (WB)
HGC-27 and HGC-27-HG cells were collected and seeded into 6-well plates. After cell adherence, the cells were treated with drugs for 24 h. Subsequently, the old medium was aspirated, and the cells were washed with PBS. Each well was lysed with 200 µL of RIPA lysis buffer (RIPA : PMSF = 100:1) on ice for 30 min, followed by centrifugation at 4 °C. After extracting the protein supernatant, the protein concentration was determined using the BCA method. Then, protein loading buffer was added to the samples, and the mixture was heated at 95 °C in a metal bath for 6 min. The prepared samples were ready for electrophoresis. The voltage was set to a constant 80 mV, and electrophoresis was stopped once the bromophenol blue reached the bottom of the glass plate. After electrophoresis, the PVDF membrane was activated with methanol, and the proteins were transferred to the membrane at 400 mA. The membrane was then blocked with 5% skim milk solution at room temperature for 2 h. After washing the membrane several times with TBST, it was incubated with primary antibodies at 4 °C for 16–24 h. Subsequently, homologous secondary antibodies were added, and the membrane was incubated at room temperature for 1–2 h. The membrane was washed again with TBST, and protein signals were visualized using chemiluminescence reagents. Finally, target proteins were analyzed using ImageJ software.
Xenotransplantation model of nude mouse
Compared to female mice, male mice exhibit less variability in hormonal fluctuations, particularly in studies involving glucose regulation, which is relevant to this research. Therefore, all mice used in this experiment were male. Male nude mice (BALB/c-nu, 14–18 g) were obtained from Hefei Qingyuan Biotechnology Co., Ltd. and acclimatized for 7 days under specific pathogen-free (SPF) conditions with a 12/12 h light-dark cycle. Nine mice were utilized in this study. Tumor volume was measured every 3 days using the formula: Volume = 0.5 × length × width2. The mice were randomly assigned to three groups: normal blood glucose, diabetic, and diabetic plus drug treatment. A diabetic mouse model was induced by administering a high-fat, high-sugar diet followed by fasting and a single intraperitoneal injection of streptozotocin (STZ). Subsequently, HGC-27 cells (5 × 106 cells in 100 µL PBS) were subcutaneously injected into the flank of each mouse. Quercetin was dissolved in injectable soybean oil. Upon the appearance of measurable tumors on day 12, the normal blood glucose and diabetic groups received 0.2 mL soybean oil every other day for a total of 20 injections, whereas the diabetic plus drug treatment group was administered 50 mg/kg quercetin in 0.2 mL soybean oil on the same schedule.
At the experiment’s conclusion, mice were euthanized via intravenous injection of 150 mg/kg sodium pentobarbital. Death was confirmed by the cessation of respiration and heartbeat, along with pupil dilation. The humane endpoint was defined as tumor volume reaching 10% of the mouse’s body weight (approximately 2000 mm3). No animals reached this endpoint prior to the experiment’s termination. Tumors were harvested for ex vivo analysis. The animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Approval No.: LISC20242443). The animal experiments were conducted in accordance with the ARRIVE guidelines. The mice were cared for and treated following the guidelines for the care of experimental animals, and euthanasia was performed upon completion of the experiments.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 10 software (version 10.1.2). All experiments were conducted with three biological replicates, and data are presented as mean ± SD. For normally distributed data, comparisons between two groups were analyzed using unpaired t-tests, while comparisons among multiple groups were performed using one-way ANOVA followed by Tukey’s post hoc test. A P-value < 0.05 was considered statistically significant.
Results
Related targets of Banzhilian on GC-diabetes
First, 9,503 genes associated with diabetes (Fig. 2A) and 12,934 genes related to stomach cancer (Fig. 2B) were collected from online databases, including GeneCard, OMIM, DrugBank, TTD, and PharmGKB. Additionally, 29 active ingredients and 202 drug targets were procured from the TCMSP database. The intersection of the aforementioned gene sets resulted in 175 target genes related to the action of Banzhilian in GC-diabetics (Fig. 2C). The interaction network of Banzhilian in anti-GC-diabetes was then constructed using Cytoscape software (Fig. 2D). Based on the network analysis, quercetin was predicted as the effective monomeric compound of Banzhilian for anti-GC-diabetes, and therefore, quercetin was selected for further experiments.
Prediction of Banzhilian’s components and targets in anti-GC-diabetes, along with GO and KEGG analysis. (A) Diabetes-related genes. (B) GC-related genes. (C) Identification of intersection targets between Banzhilian and GC-diabetes. (D) Interaction between Banzhilian’s active components and GC-diabetes pathogenic genes. (E,F) Key targets were identified using the CytoNCA plugin. (G) GO analysis. (H) KEGG enrichment analysis.
PPI network
To determine the drug action targets, the filtered PPI network was imported into Cytoscape, and CytoNCA was employed to compute the betweenness, closeness, degree, eigenvector, LAC, and network scores of the network. Five core target genes—TP53, AKT1, CCND1, JUN, and MYC—were identified as key players in Banzhilian’s action on GC-diabetes (Fig. 2E,F).
Enrichment analysis results
The Gene Ontology (GO) analysis (Fig. 2G) indicated that the therapeutic effects of Banzhilian on GC-diabetes primarily involve hormone response, response to exogenous stimuli, response to inorganic substances, and transcription factor binding. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis identified the top 20 pathways related to the treatment of GC-diabetes by Banzhilian (Fig. 2H), with pathways in cancer, lipid metabolism, and atherosclerosis being prominent. Based on these results and the core target genes identified, the PI3K/AKT signaling cascade emerged as the mechanism of action for this study.
Molecular Docking results
Molecular docking methods were utilized to assess the binding affinity of small molecule drugs to potential target proteins, providing insight into their potential therapeutic effects. Affinity, an evaluation index for the therapeutic effect, reflects how tightly ligand molecules bind to receptor proteins—lower negative affinity values indicate stronger binding. The affinity of quercetin for each key target is shown in the table, and the molecular docking results of quercetin binding to all five key targets are presented in the figure. The results demonstrate that quercetin forms hydrogen bonds with all key targets, with the strongest binding to AKT1. The molecular docking analysis suggests that quercetin may exert regulatory and therapeutic effects in cancer through these protein interactions(Table 1) (Fig. 3A,B).
Molecular docking and molecular dynamics simulation. (A) Molecular docking results were obtained using Autodock Vina software and visualized using Open-Source PyMOL(Schrodinger, LLC). (B) 2D representation of molecular docking, the 2D diagrams of molecular docking were visualized using LigPlot+ (v2.2). (C,D) Root Mean Square Deviation (RMSD) variation over time. (E) Hydrogen bond analysis.
MD simulation results
MD simulations were executed to further validate the binding affinity of quercetin to key target proteins. On the basis of the molecular docking outcomes, simulations were executed for the five protein-ligand complexes over 100 ns under ambient temperature and pressure conditions and evaluated by RMSD (Fig. 3C,D). The quercetin-protein complex began to stabilize at approximately 40 ns. The number of hydrogen bonds during the simulation aligned with the molecular docking findings, further supporting the stability of the binding system between quercetin and the target proteins (Fig. 3E). These observations suggest that the binding interactions between quercetin and the target proteins are highly stable.
Survival analysis results
The prognostic significance of five core target genes in individuals with GC was evaluated. High AKT1, TP53, and CCND1 levels were associated with shorter OS and post-progression survival (PPS) (p < 0.05), whereas elevated JUN and MYC levels correlated with prolonged OS and PPS (p < 0.05). These results demonstrate a strong correlation between the five core target genes and both OS and PPS (Fig. 4A).
Survival analysis and immunohistochemistry. (A) Impact of elevated and reduced expression patterns of five key genes on overall survival (OS) and post-progression survival (PPS) in individuals with Gastric cancer. (B) Expression levels of P53, AKT, JUN, MYC, and CCND1 in normal gastric tissues and Gastric cancer tissues. scale bar, 25 μm.
Immunohistochemical assay results
Immunohistochemical analysis (Fig. 4B) revealed that P53, AKT, and MYC protein levels in cancer tissues from patients with GC-diabetes were elevated relative to that observed in normal gastric tissues, with AKT and MYC showing significant upregulation.
Cell viability test results
CCK-8 assay outcomes indicated that quercetin markedly reduced the survival rate of HGC-27-HG cells in a time- and dose-dependent manner. The IC50 value for quercetin treatment of HGC-27-HG cells after 24 h was measured to be 57.24 µM, thus establishing 24 h as the intervention time. Low, medium, and high concentrations of quercetin were set at 20, 60, and 100 µM, respectively (Fig. 5A,B). In subsequent experiments, HGC-27, HGC-27-HG, and the low, medium, and high dose groups of HGC-27-HG were labeled as Group 1, Group 2, Group 3, Group 4, and Group 5.
Investigation of the anti-GC-diabetes effect of quercetin on cell proliferation. (A) IC50 value of quercetin on HGC-27-HG cells. (B) CCK-8 assay for HGC-27-HG cells. (C,D) Clonal formation rate of HGC-27-HG cells. (E,F) Cell proliferation activity was assessed by EDU staining. scale bar, 200 μm. (G) Cell cycle alterations in HGC-27-HG cells. (H) Cell activity was measured by AMPI staining. scale bar, 50 μm. (I) Apoptosis rate in HGC-27 cells. (J–M) Expression of apoptosis-related proteins. HGC-27, HGC-27-HG, and the low, medium, and high dose groups of HGC-27-HG were labeled as Group 1, Group 2, Group 3, Group 4, and Group 5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Effect of Quercetin on the proliferation and killing of hyperglycemic GC cells
To assess the impact of quercetin on the proliferation of HGC-27-HG cells, clonogenic assays and EdU staining were executed. The outcomes suggested that the colony formation rate of high-glucose-treated HGC-27-HG cells was increased compared to that of normal HGC-27 cells, quercetin treatment markedly decreased both colony quantity and formation efficiency of HGC-27-HG cells in a dose-dependent manner (Fig. 5C,D). EdU staining further demonstrated that a high-glucose environment promotes the entry of HGC-27 cells into the proliferative phase, while the number of proliferative HGC-27-HG cells significantly decreased after drug treatment (Fig. 5E,F), providing additional evidence that quercetin inhibits the proliferation of these cells. To corroborate these results, cell cycle analysis was performed, revealing that quercetin effectively arrested the cell cycle of HGC-27-HG cells in the G0/G1 phase (Fig. 5G).
To assess the cytotoxic effects of quercetin on HGC-27-HG cells, Calcein-AM/PI staining and apoptosis assays were conducted. The results indicated that the mortality rate of high-glucose-treated HGC-27-HG cells showed no significant difference compared to that of normal HGC-27 cells, the number of dead HGC-27-HG cells in the low-concentration treatment group showed no notable difference relative to the blank group, whereas substantial cell death was observed in the medium- and high-concentration drug treatment groups (Fig. 5H). Compared with the control group, compared to the control group, the apoptosis rate of HGC-27 cells under normal glucose conditions showed no significant difference, while the apoptosis rate in the quercetin-treated group increased, with a particularly notable rise observed in the medium- and high-concentration groups (Fig. 5I). To further investigate the pro-apoptotic effect of quercetin on diabetic GC cells, we performed Western blot analysis to detect the expression levels of key apoptosis-related proteins. The results demonstrated that compared to the untreated control, quercetin treatment significantly upregulated the expression of Bax and Caspase-3 in HGC-27-HG cells while downregulating the expression of Bcl-2. Additionally, the ratio of cleaved Caspase-3 to Caspase-3 was significantly increased, the expression levels of HGC-27 cells under normal glucose conditions also showed no significant differences. (Fig. 5J–M). These findings collectively suggest that quercetin promotes cellular apoptosis and exhibits therapeutic potential against GC under diabetic conditions.
Effect of Quercetin on migration and invasion of GC cells with high glucose
To evaluate the impact of quercetin on the migration and invasion capabilities of HGC-27-HG cells under high-glucose conditions, scratch wound healing assays and Transwell chamber assays were conducted. The experimental results revealed that both the wound healing rate and the migration and invasion abilities of high-glucose-treated HGC-27-HG cells were significantly higher than those of normal HGC-27 cells. Furthermore, in HGC-27-HG cells, after 24 h of quercetin treatment, the wound healing rate, migration ability, and invasion ability in all three drug-treated groups showed a dose-dependent decrease compared to the control group (Fig. 6A–F). To further validate our findings, we examined the expression levels of EMT-related proteins. The results demonstrated that after 24 h of quercetin treatment, the levels of Vimentin, N-cadherin, and Snail were reduced, while the expression of E-cadherin was upregulated, indicating that quercetin inhibits EMT in HGC-27-HG cells (Fig. 6G–K).
Investigation of quercetin’s anti-GC-diabetes effect on cell transfer. (A,B) Scratch healing rate in HGC-27-HG cells. scale bar, 100 μm. (C,D) Transwell migration capacity of HGC-27-HG cells. scale bar, 50 μm. (E,F) invasiveness of HGC-27-HG cells. (G–K) Expression of EMT-related proteins. (L,M) Analysis of PI3K, p-PI3K, AKT, p-AKT, and MYC protein abundance through Western blot technique. HGC-27, HGC-27-HG, and the low, medium, and high dose groups of HGC-27-HG were labeled as Group 1, Group 2, Group 3, Group 4, and Group 5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
WB results
Based on our previous analysis, we hypothesized that quercetin might inhibit HGC-27-HG cells by targeting the PI3K/AKT pathway. Therefore, we examined the expression levels of pathway-related proteins. The experimental results showed that after quercetin treatment, the protein expression levels of p-PI3K, p-AKT, and MYC were significantly reduced (Fig. 6L,M). These findings suggest that quercetin may exert its effects by inhibiting the PI3K/AKT/MYC signaling pathway, thereby inducing apoptosis and suppressing the proliferation, migration, and invasion of HGC-27-HG cells.
Results of xenotransplantation model in nude mice
STZ was rapidly injected into nude mice on an empty stomach to induce diabetes, and the blood glucose levels of diabetic mice stabilized around 20 mmol/L (Fig. 7A), with body weights lower than those of the normal blood glucose group (Fig. 7B), confirming successful diabetes modeling. HGC-27 cells were subcutaneously injected into the right abdomen of the mice. Tumor size and volume were found to be markedly smaller in the normal blood glucose and diabetes + drug treatment groups relative to the control group (Fig. 7C–F). Histological analysis using H&E staining (Fig. 7G), TUNEL staining (Fig. 7I), and Ki-67 immunohistochemistry (Fig. 7H) revealed that Ki-67 expression was lower, and the number of apoptotic cells was markedly higher in tumors from diabetes + drug treatment group relative to the control group. The findings indicate that quercetin can effectively suppress tumor proliferation and stimulate apoptosis in HGC-27-HG cells in vivo.
In vivo validation of quercetin’s effects on diabetic Gastric cancer. (A) Blood glucose change curve in mice. (B) Weight change curve in mice. (C) Tumor volume change curve in mice. (D) Representative images of individual tumors from all experimental groups. (E,F) Tumor measurements and weight determination following euthanasia. (G) H&E staining of tumor tissues. scale bar, 100 μm. (H) Immunohistochemical analysis of Ki67 expression in tumor specimens. scale bar, 100 μm. (I) TUNEL assay to detect tissue apoptosis. scale bar, 100 μm. (J) Schematic diagram illustrating quercetin’s function in treating GC-diabetes generated by Figdraw. NG, HG, and HG + Q represent the normal blood glucose group, the high blood glucose group, and the high blood glucose group treated with quercetin, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
Gastric cancer ranks as the fifth most prevalent malignancy globally, with known risk factors including Helicobacter pylori infection, advanced age, high salt intake, and diets low in fruits and vegetables43. Over the past few years, diabetes prevalence has risen annually, largely due to improvements in living standards, as well as changes in work pressures and lifestyle patterns in China. Diabetes, with its high prevalence and multiple health complications, ranks among the top ten principal causes of mortality in modern society44. In 2019, an estimated 4.2 million adults aged 20–79 died from diabetes, representing 11.3% of total mortality cases45. Diabetic patients often present with hyperglycemia, hyperinsulinemia, and insulin resistance, conditions that markedly influence tumor cell behavior. Research has demonstrated a link between type 2 diabetes and cancer through metabolic disturbances such as hyperinsulinemia and hyperlipidemia46,47. Elevated insulin levels in hyperinsulinemia activate the insulin/IGF signaling axis, which in turn stimulates the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) signaling pathways. These pathways contribute to cancer cell proliferation, survival, motility, and resistance to therapy48,49,50. The management of diabetes typically involves insulin therapy alongside lifelong administration of metformin, thiazolidinediones, and sulfonylureas. However, these interventions often provide limited efficacy in maintaining blood glucose levels and impose significant financial burdens on patients and their families51.
In this study, the active ingredient of Banzhilian and their corresponding targets were screened through NP. The analysis revealed that AKT1 is a key target gene and is one of the three isoforms of AKT (protein kinase B). Survival analysis indicated that AKT1 serves a pivotal function in the prognosis of patients with GC. Additionally, pathway enrichment examination via KEGG revealed the PI3K/AKT cascade as an essential signaling route. This pathway orchestrates numerous cellular functions, encompassing development, cell division, metabolic processes, and cell maintenance52. The PI3K complex incorporates three components: regulatory elements p85 and p55, alongside the catalytic element p110. Structural variations and target preferences subdivide PI3K into three distinct categories: I, II, and III53. Receptor tyrosine kinases convert extracellular signals into intracellular pathways, converting PIP2 to PIP354. Upon activation, PI3K recruits AKT1 to the membrane, triggering its phosphorylation and subsequent activation55. The KEGG database shows that the PI3K/AKT pathway intersects with multiple signaling cascades, influencing diverse biological activities56. The MYC protein family, consisting of MYC (c-MYC), MYCN (N-MYC), and MYCL (L-MYC)57, is also involved in regulating cellular functions. Studies have demonstrated that AKT, acting upstream of MYC, contributes to the proliferation of endometrial cancer cells58. The study conducted by Kang et al. revealed that POM121 facilitates the phosphorylation process within the PI3K/AKT signaling cascade and upregulates MYC expression. Conversely, the suppression of POM121 was found to curtail the proliferative capacity, clonogenic potential, migratory behavior, and invasive propensity of Gastric cancer (GC) cells59. The research conducted by Wei et al. demonstrated an upregulation of LPCAT1 in both LUAD tissues and cell lines, which in turn facilitates the brain metastasis of lung adenocarcinoma by activating the PI3K/AKT/MYC signaling pathway60.NP and immunohistochemical results both suggest a significant role for MYC in this study. Therefore, it was hypothesized that Banzhilian might exert its effects on GC-diabetes through the PI3K/AKT/MYC signaling pathway. Numerous studies have indicated that quercetin is present in the traditional Chinese herb Banzhilian61,62,63. Quercetin is a naturally occurring flavonoid found in a variety of plants, and it demonstrates significant inhibitory effects on multiple types of cancer64. Quercetin can induce ferroptosis in Gastric cancer cells by targeting SLC1A5 and modulating the p-Camk2/p-DRP1 and NRF2/GPX4 axes65. Additionally, it exerts inhibitory effects on prostate cancer by regulating the ROS, Akt, and NF-κB signaling pathways66. In addition, quercetin protected against cancer and chemotherapy-induced muscle mass loss through improving mitochondrial homeostatic balance67. According to the findings of Hu et al., quercetin regulates oxidative and inflammatory networks to enhance the sensitivity of pancreatic cancer cells to chemotherapy drugs68.
To validate this hypothesis, the effects of quercetin on HGC-27-HG cells were assessed through in vitro experiments. The results demonstrated a concentration-dependent inhibition of cell proliferation and metastasis, with cells remaining in the G0/G1 phase. The clonogenic ability of HGC-27-HG cells was markedly reduced. Furthermore, migration and invasion assays uncovered a decrease in the migration and invasion abilities of the cells. WB analysis showed that E-cadherin protein expression was elevated, while Vimentin, N-cadherin, Snail, p-PI3K, p-AKT, and MYC expression levels were downregulated. These findings suggest that quercetin, as an active component of Banzhilian, may play a pivotal role in the prevention and treatment of HGC-27-HG cells by inhibiting the PI3K/AKT/MYC signaling pathway (Fig. 7J). In addition, in vivo experiments confirmed that quercetin can suppress the proliferation and promote apoptosis of HGC-27-HG cells in vivo.
Banzhilian, a renowned traditional Chinese medicinal herb, is celebrated for its multifaceted therapeutic properties, including heat-clearing and detoxifying effects, promotion of blood circulation and resolution of stasis, as well as reduction of swelling and alleviation of pain. In clinical practice, it is extensively utilized for its anti-inflammatory, immunomodulatory, hepatoprotective, antioxidant, and antitumor activities. Notably, its application in antitumor therapy stands out as particularly significant. While numerous studies have documented the efficacy of Scutellaria barbata in treating a variety of cancers, its therapeutic potential in the context of cancer complicated with diabetes remains inadequately understood. The results of this study offer valuable data that support further in-depth investigation into the role of Scutellaria barbata in managing cancer concurrent with diabetes.
Conclusion and further work
In summary, this study utilized network pharmacology (NP), molecular docking, molecular dynamics (MD) simulations, and bioinformatics analysis to preliminarily elucidate the therapeutic mechanism of quercetin against HGC-27-HG cells, which was subsequently validated through in vitro and in vivo experiments. The results demonstrated that quercetin, as an active component of Banzhilian, inhibits the proliferation, clonogenicity, metastasis of HGC-27-HG cells and induces cell cycle arrest at the G0/G1 phase by downregulating the PI3K/AKT/MYC pathway. Additionally, quercetin promotes apoptosis by downregulating Bcl-2 and upregulating Bax and Cleaved Caspase-3, highlighting its anti-GC-diabetes effects. This research not only clarifies the molecular mechanism of quercetin’s anti-GC-diabetes activity but also provides experimental evidence and a solid foundation for further exploration and scientific investigation of traditional Chinese medicine in the combined treatment of cancer and diabetes.
However, constrained by the limitations of funding and time, our exploration into the molecular mechanisms underlying the treatment of diabetic Gastric cancer remains preliminary. In the future, we intend to pursue follow-up studies focusing on the following aspects: Firstly, we will employ transcriptomic and proteomic approaches to conduct high-throughput validation across each experimental group. Secondly, we will perform target gene knockout and lentiviral transfection experiments to confirm that M. schelleri exerts its inhibitory effects on diabetic Gastric cancer through the regulation of the target gene. Lastly, we will utilize Co-Immunoprecipitation (Co-IP) and Chromatin Immunoprecipitation (Ch-IP) techniques to investigate the intricate interactions between proteins and between proteins and DNA.
Data availability
The data are available to academic researchers upon request. Raw data generated and/or analysed during the current study are available from the corresponding author, upon reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 74, 229–263 (2024).
Han, B. et al. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 4, 47–53 (2024).
Digklia, A. & Wagner, A. D. Advanced Gastric cancer: Current treatment landscape and future perspectives. World J. Gastroentero. 22, 2403–2414 (2016).
Harreiter, J. & Roden, M. [Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien Klin. Wochenschr. 135, 7–17 (2023).
Lega, I. C. & Lipscombe, L. L. Review: Diabetes, obesity, and Cancer-Pathophysiology and clinical implications. Endocr. Rev. 41, 33–52 (2020).
Tseng, C. The Relationship between diabetes mellitus and Gastric cancer and the potential benefits of metformin: An extensive review of the literature. Biomolecules 11, 1022. https://doi.org/10.3390/biom11071022 (2021).
He, X. et al. Hyperglycemia induces miR-26-5p down-regulation to overexpress PFKFB3 and accelerate epithelial-mesenchymal transition in Gastric cancer. Bioengineered 13, 2902–2917 (2022).
Yu, J. et al. Hyperglycemia induces gastric carcinoma proliferation and migration via the Pin1/BRD4 pathway. Cell. Death Discov. 8, 224 (2022).
Roderburg, C., Loosen, S. H., Hoyer, L., Luedde, T. & Kostev, K. Prevalence of diabetes mellitus among 80,193 Gastrointestinal cancer patients in five European and three Asian countries. J. Cancer Res. Clin. 148, 1057–1062 (2022).
Yang, H. et al. Diabetes mellitus is associated with an increased risk of Gastric cancer: A cohort study. Gastric cancer. 23, 382–390 (2020).
Nam, S. Y., Jeong, J., Lee, W. K. & Jeon, S. W. Sex-specific effect of body mass index and fasting glucose on Gastric cancer risk and all causes mortality; A cohort study of 5.17 million. Int. J. Obes. 46, 1644–1651 (2022).
Kashihara, H. et al. The effect of Roux-en-Y reconstruction on type 2 diabetes in the early postoperative period. Anticancer Res. 38, 4901–4905 (2018).
Lee, W. et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in Gastric cancer patients with diabetes mellitus. Obes. Surg. 22, 1238–1243 (2012).
Biagioni, A. et al. Update on Gastric cancer treatments and gene therapies. Cancer Metast. Rev. 38, 537–548 (2019).
Zhao, W. et al. High glucose promotes Gastric cancer chemoresistance in vivo and in vitro. Mol. Med. Rep. 12, 843–850 (2015).
Meng, X. et al. Tumor metabolism destruction via metformin-based Glycolysis Inhibition and glucose oxidase-mediated glucose deprivation for enhanced cancer therapy. Acta Biomater. 145, 222–234 (2022).
Asghariazar, V. et al. Metformin Reverses 5-FU Chemoresistance by Downregulating DKK1, WNT5A, and ABCB1 Expressions in Gastric cancer: An Experimental and Bioinformatic Study (N-S ARCH PHARMACOL, 2025).
Newman, C., Rabbitt, L., Ero, A. & Dunne, F. P. Focus on metformin: Its role and safety in pregnancy and beyond. Drugs 83, 985–999 (2023).
Zhang, Q. et al. Berberine represses human Gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via Inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 128, 110245 (2020).
Yu, J., Ji, H., Dong, X., Feng, Y. & Liu, A. Apoptosis of human gastric carcinoma MGC-803 cells induced by a novel astragalus Membranaceus polysaccharide via intrinsic mitochondrial pathways. Int. J. Biol. Macromol. 126, 811–819 (2019).
Fu, M., Liu, Y., Cheng, H., Xu, K. & Wang, G. Coptis chinensis and dried ginger herb combination inhibits gastric tumor growth by interfering with glucose metabolism via LDHA and SLC2A1. J. Ethnopharmacol. 284, 114771 (2022).
Sun, J. et al. Chemical constituents, Anti-Tumor mechanisms, and clinical application: A comprehensive review on scutellaria Barbata. Molecules 29, 4134 https://doi.org/10.3390/molecules29174134 (2024).
Xue, S., Geng, A., Lian, T. & Liu, Y. Scutellaria Barbata D. Don inhibits cervical cancer cell proliferation, migration, and invasion via miR-195-5p/LOXL2 axis. Toxicol. Res.-UK. 11, 804–811 (2022).
Liu, L. et al. Flavonoids from scutellaria Barbata D. Don exert antitumor activity in colorectal cancer through inhibited autophagy and promoted apoptosis via ATF4/sestrin2 pathway. Phytomedicine 99, 154007 (2022).
Sheng, D., Zhao, B., Zhu, W., Wang, T. & Peng, Y. Scutellaria Barbata D.Don (SBD) extracts suppressed tumor growth, metastasis and angiogenesis in prostate cancer via PI3K/Akt pathway. BMC Complement. Med. 22, 120 (2022).
Shim, J. H., Gim, H., Lee, S. & Kim, B. J. Inductions of caspase-, MAPK- and ROS-dependent apoptosis and chemotherapeutic effects caused by an ethanol extract of scutellaria Barbata D. Don in human gastric adenocarcinom cells. J. Pharmacopunct. 19, 129–136 (2016).
Li, W. & Xiao, H. Scutellaria Barbata D. Don polysaccharides inhibit high Glucose-Induced proliferation and angiogenesis of retinal vascular endothelial cells. Diabet. Metab. Synd. Ob. 14, 2431–2440 (2021).
Wishart, D. S. et al. DrugBank 5.0: A major update to the drugbank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Liu, X. et al. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 38, W609–W614 (2010).
Zhou, Y. et al. TTD: Therapeutic target database describing target druggability information. Nucleic Acids Res. 52, D1465–D1477 (2024).
Ru, J. et al. TCMSP: A database of systems Pharmacology for drug discovery from herbal medicines. J. Cheminform.. 6, 13 (2014).
Szklarczyk, D. et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Laskowski, R. A. & Swindells, M. B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Sousa Da Silva AW and Vranken WF. ACPYPE - AnteChamber python parser interface. BMC Res. Notes. 5, 367 (2012).
Kagami, L., Wilter, A., Diaz, A. & Vranken, W. The ACPYPE web server for small-molecule MD topology generation. Bioinformatics 39, btad350 https://doi.org/10.1093/bioinformatics/btad350 (2023).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Hess, B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 4, 116–122 (2008).
Humphrey, W., Dalke, A. & Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet 396, 635–648 (2020).
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pr. 157, 107843 (2019).
Saeedi, P. et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pr. 162, 108086 (2020).
Samuel, S. M., Varghese, E., Varghese, S. & Busselberg, D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat. Rev. 70, 98–111 (2018).
Garcia-Jimenez, C., Garcia-Martinez, J. M., Chocarro-Calvo, A. & De la Vieja, A. A new link between diabetes and cancer: Enhanced WNT/beta-catenin signaling by high glucose. J. Mol. Endocrinol. 52, R51–R66 (2014).
Bell, J. L. et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of cancer progression? Cell. Mol. Life Sci. 70, 2657–2675 (2013).
Novosyadlyy, R. et al. Insulin-mediated acceleration of breast cancer development and progression in a Nonobese model of type 2 diabetes. Cancer Res. 70, 741–751 (2010).
Nelson, E. R. et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342, 1094–1098 (2013).
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: A review of current evidence. Diabetologia 62, 3–16 (2019).
Yang, J. et al. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 18, 26 (2019).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 (2006).
Bader, A. G., Kang, S., Zhao, L. & Vogt, P. K. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer. 5, 921–929 (2005).
Tewari, D., Patni, P., Bishayee, A., Sah, A. N. & Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 80, 1–17 (2022).
Shorning, B. Y., Dass, M. S., Smalley, M. J. & Pearson, H. B. The PI3K-AKT-mTOR pathway and prostate cancer: At the crossroads of AR, MAPK, and WNT signaling. Int. J. Mol. Sci. 21, 4507 https://doi.org/10.3390/ijms21124507 (2020).
Brodeur, G. M., Seeger, R. C., Schwab, M., Varmus, H. E. & Bishop, J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–1124 (1984).
Hsin, I., Shen, H., Chang, H., Ko, J. & Wang, P. Suppression of PI3K/Akt/mTOR/c-Myc/mtp53 positive feedback loop induces cell cycle arrest by dual PI3K/mTOR inhibitor PQR309 in endometrial cancer cell lines. Cells-Basel 10, 2916 https://doi.org/10.3390/cells10112916 (2021).
Kang, C. et al. POM121 promotes the proliferation and metastasis of Gastric cancer via PI3K/AKT/MYC pathway. Am. J. Cancer Res. 13, 485–497 (2023).
Wei, C. et al. LPCAT1 promotes brain metastasis of lung adenocarcinoma by up-regulating PI3K/AKT/MYC pathway. J. Exp. Clin. Cancer Res. 38, 95 (2019).
Zhang, J. et al. Exploration of the effect and mechanism of scutellaria Barbata D. Don in the treatment of ovarian cancer based on network pharmacology and in vitro experimental verification. Medicine 102, e36656 (2023).
Shi, H. et al. Molecular assessment of scutellaria barbata d. don in the treatment of nasopharyngeal carcinoma based on network pharmacology and experimental verification. Evid-Based Compl. Alt. 2022, 1988378 (2022).
Hou, C. et al. Network-based pharmacology-based research on the effect and mechanism of the Hedyotis diffusa-Scutellaria Barbata pair in the treatment of hepatocellular carcinoma. Sci. Rep.-UK. 14, 963 (2024).
Deepika & Maurya, P. K. Health benefits of quercetin in age-related diseases. Molecules 27, 2498 https://doi.org/10.3390/molecules27082498 (2022).
Ding, L. et al. Quercetin induces ferroptosis in Gastric cancer cells by targeting SLC1A5 and regulating the p-Camk2/p-DRP1 and NRF2/GPX4 axes. Free Radical Bio Med. 213, 150–163 (2024).
Ward, A. B. et al. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 16, 108 (2018).
VanderVeen, B. N. et al. Quercetin improved muscle mass and mitochondrial content in a murine model of cancer and chemotherapy-induced cachexia. Nutrients 15, 102 https://doi.org/10.3390/nu15010102 (2022).
Hu, Y., Li, R., Jin, J., Wang, Y. & Ma, R. Quercetin improves pancreatic cancer chemo-sensitivity by regulating oxidative-inflammatory networks. J. Food Biochem. 46, e14453 (2022).
Acknowledgements
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This work was supported by the Anhui Province clinical key specialty construction project(025), Anhui Provincial Key Research Project of Health and Wellness (AHWJ2022b079, AHWJ2023A10093), Key Projects of Anhui Provincial Department of Education’s Scientific Research Program for Higher Education Institutions (2022AH051171), National Natural Science Foundation of China (82403333), Natural Science Foundation project of Anhui Province (2408085QH271), Anhui Medical University scientific research level improvement plan (2022xkjT028), Health research project of Anhui Province (AHWJ2023A30047).
Author information
Authors and Affiliations
Contributions
Conceptualization: HKC. Methodology: HKC, HS, XHL, RF, JJC, YXC. Software: HKC, HS, ZLZ, XLL, SWW. Formal analysis: HKC, HS, WGZ, WLY. Investigation: HKC, ZX, HYY. Data curation: HKC, WJ, YBH, CQG. Project administration: JGJ. Writing–original draft preparation: HKC, WW. Writing–review and editing: HKC, BC, GDC. Visualization: HKC.Funding acquisition: HKC, JGJ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Approval No.: LISC20242443). The animal experiments were conducted in accordance with the ARRIVE guidelines. The mice were cared for and treated following the guidelines for the care of experimental animals, and euthanasia was performed upon completion of the experiments. All authors read and approved the manuscript. The processing of volunteer tissue samples has been approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical University (BYEFY(2022) Ethics Review No. 29 A) and strictly adheres to the ethical standards of the Declaration of Helsinki. Informed consent was obtained from all participants and/or their legal guardians, and written informed consent forms were signed.
Consent for publication
All authors gave their consent for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, H., Song, H., Zhou, W. et al. Exploring the active ingredients of Banzhilian and its mechanism of action on diabetic Gastric cancer based on network pharmacology. Sci Rep 15, 14808 (2025). https://doi.org/10.1038/s41598-025-98214-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98214-6