Abstract
Challenges associated with the cathode material of Lithium–oxygen (Li–O2) battery, particularly the sluggish kinetics of oxygen reduction reaction (ORR) and oxygen evolution reaction, as well as the accumulation of discharge product Li2O2, have hindered its further advancement. Here, an in-situ synthesis strategy was adopted to load a bimetallic Ni–Co metal–organic framework (Ni/Co-MOF) onto MXene (Ti3C2) layers. Subsequently, a free-standing and flexible Ni/Co-MOF@Ti3C2 hybrid membrane is prepared via a layer-by-layer self-assembly method, specifically designed for efficient ORR and as a cathode for Li–O2 batteries. The Ni/Co-MOF@Ti3C2 hybrid membrane integrates the high conductivity, unique two-dimensional layered structure, and excellent mechanical properties of Ti3C2 with the bimetallic active sites of Ni/Co-MOF, exhibiting remarkable ORR catalytic activity in an O2-saturated 1 M LiTFSI electrolyte. The structural characteristics of the hybrid membrane provide smoother expansion pathways for Li+ and O2, effectively promoting the deposition and decomposition of Li2O2. This not only enhances the electrochemical performance of the Li–O2 battery but also overcomes the inherent limitations of traditional slurry-based cathode preparation methods. Experimental results demonstrate that Li–O2 batteries utilizing the Ni/Co-MOF@Ti3C2 hybrid membrane as the cathode achieve an ultra-high capacity of 36,125 mAh/g at a current density of 1000 mA/g, while exhibiting excellent cycle stability (271 cycles with a limited capacity of 1000 mAh/g at 1000 mA/g) and outstanding rate performance. The hybrid membrane’s capacity and cycle performance can be further optimized by controlling its thickness. These promising results offer novel insights into the innovative design of air cathodes for metal–air batteries, and the proposed method provides a new route for the manufacture of high-performance battery cathodes.
Similar content being viewed by others
Introduction
The enhancement of power battery performance has become pivotal in driving advancements in electric vehicles, energy storage systems, and other related fields with the global community’s sustained focus and investment in renewable energy technologies1,2,3. In recent years, the exploration of next-generation high-energy battery technologies has emerged as a central focus as the lithium-ion battery market has gradually reached saturation4,5. Among these, lithium–oxygen (Li–O2) batteries have garnered significant attention due to their exceptional theoretical gravimetric energy density6,7,8. The working principle of Li–O2 batteries is based on redox reactions occurring in the cathode region, oxygen reacts with Li+ under the catalysis of a catalyst to from lithium peroxide (Li2O2) during discharge, known as the oxygen reduction reaction (ORR). Conversely, Li2O2 decomposes into lithium ions and oxygen during charging, referred to as the oxygen evolution reaction (OER). However, the kinetic processes of ORR and OER on the cathode are relatively slow, and the accumulation of Li2O2 severely limits key performance indicators of Li–O2 batteries, such as capacity, rate capability, and charge–discharge polarization9,10,11,12. Furthermore, the insulating and insoluble nature of Li2O2 leads to its deposition within cathode pores after prolonged discharge, further hindering diffusion, increasing electrode polarization and impedance, and thereby shortening the battery’s cycle life13,14. The key to overcoming these challenges lies in the innovation and optimization of air cathode.
Traditional air cathode materials include carbon materials15,16, precious metals and alloys17,18, and metal oxides19,20. But these materials possess significant drawbacks, such as instability, high cost, and limited catalytic activity. In recent years, two-dimensional (2D) layered materials have garnered considerable attention due to their exceptional physical and chemical properties21,22,23. As an emerging 2D material, MXene features a symmetric hexagonal lattice and metallic properties, exhibiting excellent conductivity, tunable bandgap, high carrier mobility, good mechanical properties, and thermal conductivity24,25. These characteristics make MXene an ideal choice for a new generation of energy storage and energy conversion materials. MXene serves as a cathode material in Li–O2 batteries, with its high surface area-to-volume ratio providing a large reaction interface26,27,28. The 2D channels enable high and controllable ion conductivity and oxygen diffusion, and the interlayer spacing can be adjusted to accelerate the rapid and reversible transport of lithium ions. Additionally, MXene is easy to process and can be composited with other materials to further enhance the material’s gravimetric energy density. However, MXene faces severe self-stacking issues during membrane formation similar to other 2D materials, the strong van der Waals forces and hydrogen bonding interactions between adjacent MXene layers cause single-component MXene nanosheets to easily restack29,30, significantly impeding ion transport channels and drastically reducing their specific surface area and available active sites.
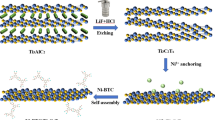
As material requirements continue to increase, single materials often fail to meet reaction demands. Composites of different materials can often produce a “1 + 1 > 2” effect, but it is crucial to consider the characteristics of the two materials to ensure they complement each other. Metal–organic frameworks (MOFs) are porous network materials with large specific surface areas, unique porosity, and ease of modification formed through the coordination self-assembly of metal ions and organic ligands31,32. MOFs possess different types of catalytic sites, providing the inherent advantages of homogeneous and heterogeneous catalysts33. Based on our group’s previous work and understanding of cathode catalysts and their structures and functions in Li–O2 batteries33, a bimetallic Ni, Co-based MOF material (Ni/Co-MOF, as shown in Fig. 1) is introduced here. Through the in-situ self-assembly properties of MOFs, Ni/Co-MOF can be loaded onto MXene layers, effectively preventing the self-restacking of MXene layers, significantly increasing the interlayer spacing of MXene, and providing faster Li+ and O2 diffusion channels. On the other hand, as bimetallic centers, Ni and Co introduce more active sites, enhancing catalytic performance and promoting the rapid and reversible formation and decomposition of Li2O2.
Diagrammatic representation of the fabrication approach for a Li–O2 battery utilizing a Ni/Co-MOF@Ti3C2 membrane as the cathode. (a) Crafting a self-supporting, flexible membrane as the cathode through a layer-by-layer deposition technique. (b) Construction of the Li–O2 battery with Ni/Co-MOF@Ti3C2 membranes serving as the cathode component. (c) The operational principle during the discharge phase of Li–O2 batteries.
In this work, Ni/Co-MOF particles were loaded onto the surface of MXene sheets through the in-situ self-assembly properties of MOFs, and a free-standing flexible Ni/Co-MOF@MXene hybrid membrane was prepared based on a layer-by-layer stacking self-assembly strategy. The resulting Ni/Co-MOF@MXene effectively alleviates the self-stacking problem of MXene and exhibits excellent ORR performance and a high specific surface area. Li–O2 batteries assembled based on the Ni/Co-MOF@MXene as cathode exhibit ultra-high capacity and excellent cycle stability, along with superior rate performance. Furthermore, the capacity and cycle performance of the assembled Li–O2 batteries can be regulated by controlling the thickness of the Co-MOF/MXene membrane. The key to this method lies in the development of a novel cathode structure that effectively utilizes the in-situ loading of bimetallic Ni/Co-MOF with abundant active sites and special structures onto MXene nanosheets, thereby increasing the interlayer spacing of the MXene layered structure and inhibiting the top-down restacking of MXene nanosheets. The research ideas and preparation methods in this study can also be extended to the preparation of other catalysts and batteries, providing valuable insights for the design of efficient catalysts and the development of novel battery structures.
Materials and methods
Materials
All chemicals were purchased directly and utilized without further purification. Ti3AlC2 powder (98%), N,N-dimethylformamide (DMF), ethanol (98%), and polypropylene membrane were from Aladdin. LiCl (99%), LiF (99%), Ni(NO3)2·6H2O, Co(NO3)2·6H2O (both 99%) and terephthalic acid (TPA, 98%) were from Alfa Aesar. HCl was from Beijing Chemical Plant.
Synthesis of 2D Ti3C2 layer
The preparation of layer-shaped Ti3C2 is achieved through etching and exfoliation of Ti3AlC2 powder using HCl/LiF34. Specifically, 20 mL of HCl solution is mixed with 1.56 g of LiF in a polytetrafluoroethylene beaker. 1 g of Ti3AlC2 powder is added in portions under continuous stirring. The entire beaker is then placed in a water bath set at 405 K, and stirring for 48 h to ensure thorough completion of the etching and exfoliation reaction. Centrifugation is performed three times at 8000 rpm using 1 M HCl solution and 1 M LiCl solution sequentially to purify the product. Multiple centrifugation washes with deionized water are conducted until the pH approaches neutrality (approximately 6). A dark green Ti3C2 suspension can be observed in the centrifuge tube. The supernatant suspension is repeatedly collected and subjected to ultrasonic treatment for 12 h to exfoliate and better disperse the Ti3C2 layers within the suspension, ultimately yielding a high-quality dispersion of Ti3C2 layers.
Synthesis of Ni/Co-MOF@Ti3C2
Take 20 mL of the prepared Ti3C2 layer dispersion and add 20 mL anhydrous ethanol, 20 mL of DMF. Subject the mixture to ultrasonic treatment to ensure homogeneous blending. Sequentially add 165 mg Ni(NO3)2·6H2O, 165 mg of Co(NO3)2·6H2O salts and 0.2 g TPA to fully dissolve under stirring. Transfer the resulting mixture to a reactor and heat it to 423 K for a reaction period of 18 h. After naturally cooling to room temperature, centrifuge to collect the solid product, and wash the solid product three times with DMF to remove adherent impurities and unreacted substances, followed by washes with anhydrous ethanol. Place the washed solid in a vacuum oven for drying for 24 h to obtain the Ni/Co-MOF@Ti3C2 powder.
As experimental comparisons, two additional materials were prepared: Ni/Co-MOF without the addition of Ti3C2 nanosheet dispersion and Co-MOF@MXene without the addition of Ni(NO3)2·6H2O.
Preparation of free-standing Ni/Co-MOF@Ti3C2 hybrid membrane
The flexible, self-supporting Ni/Co-MOF@Ti3C2 hybrid membrane was using a hierarchical self-assembly technique. Ni/Co-MOF@Ti3C2 powder is added to an appropriate amount of water and subjected to ultrasonic treatment for 2 h to ensure a homogeneous dispersion. As illustrated in Figs. S1, S2 and S3, a vacuum filtration setup is utilized for the preparation of the Ni/Co-MOF@Ti3C2 hybrid membrane. A hydrophilic polypropylene membrane is placed inside the filtration setup, and the prepared Ni/Co-MOF@Ti3C2 dispersion is poured into a sand-core funnel. The vacuum pump is activated to begin the suction filtration process. After completion of the filtration, the system is allowed to sit for a period to facilitate membrane stabilization and curing. The Ni/Co-MOF@Ti3C2 membrane is then removed from the polypropylene support membrane and dried under vacuum conditions, yielding a flexible, self-supporting Ni/Co-MOF@Ti3C2 hybrid membrane.
Materials characterizations
Structural analyses of the MOF, Ti3C2, and Ni/Co-MOF@Ti3C2 hybrid membranes were conducted using an X-ray diffractometer (XRD) equipped with a Cu Kα radiation source (model Bruker D8 ADVANCE, the 2θ angle range was set from 5° to 50° with a step size of 0.05°). N2 adsorption–desorption isotherms of the materials were measured on an Autosorb-iQ-MP automated gas sorption analyzer from Quantachrome Instruments. Prior to the adsorption and desorption tests, the samples were degassed at 423 K for 12 h. Transmission electron microscopy (TEM) using a Tecnai G2 20 S-TWIN (FEI Company) was employed to observe the morphology and size of the two-dimensional nanosheets, as well as the interlayer spacing of the membranes. Additionally, scanning electron microscopy (SEM) with a JSM-7800F (JEPL Ltd., Japan) was used to analyze the morphology of the nanosheets and membranes. To obtain clear SEM images, the samples were brittle-fractured in liquid nitrogen before observation. X-ray photoelectron spectroscopy (XPS) measurements were performed using an ESCALAB 250 instrument (Thermo VG, USA).
Assembly and performance testing of Li–O2 batteries
A modified CR2025 coin cell (with a drilled cathode case) was employed to assemble a Li–O2 battery in this study, The separator (Whatman) was pre-dried in a vacuum oven at 423 K for 12 h. The prepared Ni/Co-MOF@Ti3C2 hybrid membrane cathode, lithium metal anode, and electrolyte composed of 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in triethylene glycol dimethyl ether (TEGDME) were assembled into the battery inside an Ar-filled glovebox. After sealing, the battery was removed from the glovebox and filled with pure O2. After a resting period of 3 h, electrochemical testing was conducted.
Battery performance testing was carried out using the CT2001A battery testing system produced by Wuhan LAND Electronics Co., Ltd. The charge–discharge voltage range was set between 2.0 and 4.5 V (vs. Li/Li+), with a current density of 1000 mA/g. During the cycling performance test, the specific charge–discharge capacity was limited to 1000 mAh/g, and the current density was maintained at 1000 mA/g.
Density functional theory (DFT) calculations
DFT calculations were conducted in this study using the Vienna Ab initio Simulation Package (VASP) to delve into the electronic structure characteristics and catalytic mechanisms of Ni/Co-MOF/Ti3C2 composites as cathodes in Li–O2 batteries. The Perdew–Burke–Ernzerhof (PBE) exchange functional within the generalized gradient approximation (GGA) framework was chosen to accurately simulate the interactions between electrons, based on its proven accuracy and reliability across various material systems34,35,36. Notably, the spin-polarization effect was neglected in the calculations due to its negligible impact on the total energy of the system. In terms of computational parameters, a cutoff energy of 420 eV was set for the plane-wave basis, and the Monkhorst–Pack method was employed for Brillouin zone integration with a 5 × 5 × 1 k-point grid to ensure precision37. During geometric structure optimization, stringent convergence criteria were established, requiring the energy of all atoms to converge to 10−4 eV and forces to converge to 0.02 eV/Å or tighter, thereby ensuring the stability of the obtained structures. The Heyd–Scuseria–Ernzerhof (HSE) hybrid density functional method was further adopted to provide a more detailed depiction of the electronic structure and calculate the density of states (DOS), implemented through the Quantum ESPRESSO software package. Additionally, the Bader charge analysis method was utilized to delve into the charge transfer mechanisms within the composite material, which is crucial for understanding electron flow and the role of active sites during catalysis.
Results and discussion
Design strategy for the Li–O2 battery cathode based on Ni/Co-MOF@Ti3C2 membrane
The preparation of the Ni/Co-MOF@Ti3C2 hybrid membrane and the strategy for fabricating the Ni/Co-MOF@Ti3C2 hybrid membrane-based Li–O2 battery cathode are shown in Fig. 1. In Li–O2 batteries, the performance of the air cathode plays a crucial role in the rates of the ORR and OER, as well as the effective deposition and decomposition of reaction products. Constructing an air cathode structure that not only ensures rapid O2 and lithium-ion transport but also provides sufficient space to accommodate a large amount of discharge products has become the focus of current research. Traditional methods typically rely on coating a slurry containing binders and conductive agents onto carbon paper or carbon cloth to form the air cathode, which has several limitations, such as the surface of the air cathode being easily blocked by the binder, that hinders oxygen transport; uneven dispersion of active materials, which affects catalytic efficiency; and settlement of active materials, leading to a decline in battery performance over time. To address these issues, this work proposes an innovative solution: preparing a binder-free, free-standing air cathode (Fig. S4). This cathode adopts a layered stacking structure that not only exhibits excellent ORR/OER catalytic activity but also significantly improves the diffusion channels for O2 and Li+ through carefully designed stacking, while providing maximum space to accommodate the accumulation of discharge products. This effectively alleviates the problem of battery performance degradation caused by the accumulation of reaction products. In terms of material selection, we introduced bimetallic Ni/Co-MOF as the active material and compounded it with Ti3C2 to form the Ni/Co-MOF@Ti3C2 structure. MXene provides robust structural support for the air cathode as a two-dimensional material with high conductivity and good mechanical properties. The introduction of Ni/Co-MOF further enhances catalytic performance through its abundant pore structure and bimetallic active sites. More importantly, the in-situ loading of Ni/Co-MOF not only increases the interlayer space of Ti3C2, facilitating rapid transport of oxygen and Li+, but also introduces additional active sites, improving the efficiency of catalytic reactions. At the same time, this free-standing stacking structure allows the active materials to be more uniformly distributed within the cathode, avoiding issues of settlement and blockage.
Materials characterization
The overall morphology and microstructure of Ni/Co-MOF@Ti3C2 nanosheets were observed and analyzed using SEM, TEM, AFM, and EDS characterization techniques. Figure S5 displays the SEM images of Ti3C2 samples prepared through the HF/LiF method. The Al element in Ti3AlC2 was successfully removed after HF etching, forming Ti3C2 of approximately 1 μm with a loosely stacked and folded structure, which provides an ideal substrate for the in-situ loading of Ni/Co-MOF. It is evident that a uniform layer of Ni/Co-MOF particles has been successfully loaded onto the surface of Ti3C2 layer by observing Ni/Co-MOF@Ti3C2 nanosheets at different angles (Fig. 2a,b). An in-situ self-assembly technique was adopted to directly grow Ni/Co-MOF particles on the surface of Ti3C2 when preparing Ni/Co-MOF@Ti3C2 composite material. This growth method enables a tight and uniform combination between Ni/Co-MOF and Ti3C2, which not only increases the interlayer spacing but also promotes the rapid transport of electrons and ions. Since Ni/Co-MOF is loaded onto Ti3C2 nanosheets through in-situ growth, the binding between them is tight and uniformly distributed, which contributes to enhancing the overall performance and stability of the material. The TEM image (Fig. 2c) reveals the lattice fringes of Ni/Co-MOF and the layered structure of Ti3C2, further confirming the successful loading of Ni/Co-MOF onto Ti3C2 nanosheets and showcasing their good crystallinity and layered structure. Figure 2d presents the AFM image of Ni/Co-MOF@Ti3C2 nanosheets, indicating a thickness of 2 μm, which facilitates the diffusion and transport of Li+ and O2 in Li–O2 batteries while ensuring the mechanical strength and stability of the material. Figure 2e and f show the cross-sectional and top-view SEM images of the Ni/Co-MOF@Ti3C2 hybrid membrane, respectively. The top-view image reveals that the surface of the hybrid membrane is composed of nanosheets of different sizes stacked together, forming a microstructure with folds and tight connections. This structure helps increase the specific surface area and active sites of the material, enhancing the electrochemical performance of Li–O2 batteries. The cross-sectional image clearly shows the well-arranged layered structure (as shown in Fig. 2g), further proving the uniform distribution and good stacking of Ni/Co-MOF@Ti3C2 nanosheets within the membrane, which facilitates the insertion and extraction of lithium ions in Li–O2 batteries, improving the charge–discharge performance and cycle stability of the battery. Figure 2h and i display the EDS mappings of Co and Ni elements in the cross-sectional image of the Ni/Co-MOF@Ti3C2 hybrid membrane. The mapping results indicate that Ni/Co-MOF is uniformly distributed within the hybrid membrane, facilitating the full exposure of bimetallic active sites.
The XRD pattern of Ni/Co-MOF (Fig. 3a) aligns well with the previously reported structure38, which exhibiting intense and sharp characteristic peaks, indicative of the high crystallinity of Ni/Co-MOF. Pure Ti3C2 displays a strong diffraction peak at 6.7°, corresponding to an interlayer spacing of 1.32 nm characteristic of its typical structure. Notably, the XRD pattern of the Ni/Co-MOF@Ti3C2 hybrid membrane not only retains the crystalline structure features of Ni/Co-MOF intact but also clearly shows the characteristic peaks of Ti3C2, confirming the in-situ loading and combination of the two components. Figure S6 further presents the small-angle XRD patterns of Ni/Co-MOF@Ti3C2 and Ti3C2. Analysis reveals that upon successful loading of Ni/Co-MOF, the interlayer spacing of the Ni/Co-MOF@Ti3C2 hybrid membrane significantly increases to 1.49 nm, much larger than the 1.32 nm of the pure Ti3C2 membrane. This expansion of interlayer spacing is crucial for the diffusion and transport of O2 and Li+ during charging-discharging processes, enhancing the accessibility of electrolyte ions to the Ti3C2 nanosheets and laying the foundation for optimized electrochemical performance. Comparative tests were conducted on the N2 adsorption–desorption curves of pure Ti3C2 membrane and Ni/Co-MOF@Ti3C2 hybrid membrane, with the specific surface areas calculated as shown in Fig. 3b. The specific surface area of the pure Ti3C2 membrane is 97 m2/g, while that of the Ni/Co-MOF@Ti3C2 hybrid membrane is as high as 232 m2/g. This substantial increase is primarily attributed to the large specific surface area of Ni/Co-MOF itself (1372 m2/g, Fig. S7) and the effective inhibition of self-stacking of Ti3C2 by Ni/Co-MOF, which increases the distance between Ti3C2 lamellae. As the air cathode of Li–O2 batteries, the high-specific-surface-area Ni/Co-MOF@Ti3C2 hybrid membrane can provide more channels for the diffusion of Li+ and O2, increasing the reaction interface and the number of active sites while promoting the accumulation and decomposition of the reaction product Li2O2, thereby significantly enhancing the electrochemical performance of Li–O2 batteries. Additionally, the N2 adsorption–desorption curve of the Ni/Co-MOF@Ti3C2 hybrid membrane exhibits a type IV isotherm accompanied by an H3 hysteresis loop, indicating the presence of slit-like pores in the hybrid membrane, which further confirms the effective regulation of the interlayer spacing of the Ni/Co-MOF@Ti3C2 hybrid membrane by the incorporation of Ni/Co-MOF. Figure S8 demonstrates the difference in O2 adsorption performance between Ti3C2 and Ni/Co-MOF@Ti3C2, where the O2 adsorption capacity of pristine Ti3C2 is 1.42%, while Ni/Co-MOF@Ti3C2exhibits an enhanced O2 adsorption capacity of 2.55%. This enhancement is primarily attributed to the increased interlayer spacing and significantly larger specific surface area of the Ni/Co-MOF@Ti3C2 composite, which provides more adsorption sites and thus enhances the adsorption capacity for O2.
XPS was employed to conduct an in-depth investigation of the chemical composition and surface electronic states of the Ni/Co-MOF@Ti3C2 hybrid membrane (Fig. 3c) to unravel the interaction mechanism between Ni/Co-MOF and Ti3C2 nanosheets, the results indicate that the hybrid membrane is primarily composed of Ni, Co, O, Ti, and C elements. The high-resolution XPS spectra of Ni 2p and Co 2p (Fig. 3d,e) show that the Ni and Co elements in Ni/Co-MOF exist in the forms of Ni2+, Ni3+, and Co2+, Co3+, respectively. Notably, some of the divalent Ni and Co are oxidized to trivalent states, a transformation crucial for enhancing the catalytic performance of Ni/Co-MOF. Specifically, trivalent Ni and Co serve as active sites, accelerating the reduction rate of O2 during the ORR, thereby improving the energy conversion efficiency of Li–O2 batteries. Figure 3f displays the high-resolution XPS spectrum of the Ti 2p energy level in Ni/Co-MOF@Ti3C2 hybrid membrane. The Ti 2p energy level can be divided into Ti–C bonds from the main structure of Ti3C2 and Ti–O bonds on the surface of Ti3C2. The presence of Ti–O bonds may be due to reactions between the Ti3C2 surface and O2 in the air during preparation. However, this surface oxidation does not adversely affect the overall catalytic performance of the Ni/Co-MOF@Ti3C2hybrid membrane and may instead enhance ORR activity by providing additional active sites.
Performance testing of ORR
The electrocatalytic activity of various materials towards ORR was evaluated in O2-saturated 1 M LiTFSI electrolyte under the conditions of a scan rate of 5 mV/s and an electrode rotation speed of 900 rpm. Figure 4a displays the linear sweep voltammetry (LSV) curves for four materials: Ti3C2, Ni/Co-MOF@Ti3C2, Ni/Co-MOF, and Co-MOF. The cathodic currents of these materials all increased with potential within the specified potential range, indicating that they all possessed a certain degree of ORR catalytic capability. In addition to the LSV curves, the half-wave potential (E1/2) was determined for each material. In an O2-saturated 1 M LiTFSI electrolyte, with a scan rate of 5 mV/s and an electrode rotation speed of 900 rpm, the half-wave potential of Ni/Co-MOF@Ti3C2 is 2.46 V, while that of Ti3C2 is 2.41 V. This data directly indicates that Ni/Co-MOF@Ti3C2 exhibits higher catalytic activity during the ORR process compared to Ti3C2. The electrocatalytic activity test of the commercial catalyst Pt/C has also been added, and the results are shown in Fig. S9. It can be seen that the ORR catalytic capabilities of Ti3C2 and Ni/Co-MOF@Ti3C2 prepared in this work surpass that of the commercial Pt/C catalyst. This superior performance is mainly attributed to the synergistic effect of Ti3C2 loaded with oxygen-rich defective TiO2 and the Co–N–C catalyst, which provide abundant active sites and enhance the electronic structure for ORR. The Tafel plots in Fig. 4b further elucidate the kinetic characteristics of these materials. The Tafel slope of Ni/Co-MOF@Ti3C2 (182 mV/dec) was significantly lower than that of the Ti3C2 (344 mV/dec), suggesting that Ni/Co-MOF@Ti3C2 exhibits more efficient and faster kinetics during the ORR process. This not only demonstrates the superiority of Ni/Co-MOF@Ti3C2 as an ORR catalyst but also reveals the significant improvement in Li2O2 deposition kinetics due to its unique structure. Notably, although Ni/Co-MOF itself exhibited the highest kinetic efficiency, the composite of Ni/Co-MOF with Ti3C2 further enhanced the catalytic performance. This is attributed to the loading effect of Ni/Co-MOF that significantly boosted the catalytic activity of Ti3C2. The bimetallic Ni/Co-MOF displayed higher catalytic activity compared to the monometallic Co-MOF, primarily benefiting from the introduction of Ni active sites, which synergized with Co to enhance the adsorption and reduction capabilities of O2. Furthermore, the unique structure of Ni/Co-MOF@Ti3C2 provided abundant exposed active sites, which not only facilitated the transport of electrons and ions but also simplified the electrocatalytic kinetic process. The power density curves in Fig. 4c further confirm the excellent catalytic performance of Ni/Co-MOF@Ti3C2, as it exhibited a higher onset potential and peak current density compared to the pure Ti3C2. Figure 4d presents the ORR LSV curves of Ni/Co-MOF@Ti3C2 at different electrode rotation speeds. The current density of Ni/Co-MOF@Ti3C2 increased linearly with voltage within the voltage range of 2.7–2.9 V, indicating that the ORR was primarily controlled by kinetics at this stage. However, the current density exhibited a plateau, and the limiting diffusion current density increased with electrode rotation speed within the voltage range of 2.2–2.6 V, suggesting that the ORR process transitioned to a diffusion-controlled step at this stage. At higher scan rates, the ORR process becomes more diffusion-controlled, leading to an increase in current density with the scan rate. Meanwhile, the formation and release processes of the electric double-layer capacitance at high scan rates may result in the appearance of current peaks. Meanwhile, the ORR stability of Ni/Co-MOF@Ti3C2 material test has been supplemented to comprehensively evaluate the ORR performance. The results are shown in Fig. S15. After a long-term test (50 h), the ORR activity retention rate of Ni/Co-MOF@Ti3C2 is 87.7%. This result fully demonstrates that the material has excellent stability during the ORR process. In this work, the ORR process is mainly influenced by both diffusion control and kinetic control. The metal active centers on the surface of the Ni/Co-MOF@Ti3C2 hybrid film, acting as a catalyst, play a crucial role in the adsorption and reduction of oxygen. Therefore, it can be infer that during the ORR process catalyzed by the Ni/Co-MOF@Ti3C2 hybrid film, the surface-adsorption pathway may dominate. However, this does not mean that the solution-mediated pathway does not contribute; it is just that, relatively speaking, the contribution of the surface-adsorption pathway is more significant. The superior ORR activity of Ni/Co-MOF@Ti3C2 as a cathode material for Li–O2 batteries is primarily attributed to two aspects: first, the open Ni and Co metal sites in Ni/Co-MOF enhance the adsorption and reduction efficiency of O2; second, the unique structure of Ni/Co-MOF@Ti3C2 not only provides abundant active sites but also optimizes the transport paths for electrons and ions, thereby ensuring an efficient electrocatalytic kinetic process.
Performance testing of Li–O2 batteries
Li–O2 batteries were assembled using Ti3C2 and Ni/Co-MOF@Ti3C2 hybrid membranes with a thickness of 10 μm as cathode materials. The Ni/Co-MOF@Ti3C2 hybrid membrane was employed in these assemblies with weighing 4.8 mg and possessing a density of 3.13 g/cm3. All performance tests of the Li–O2 batteries were repeated at least three times to ensure data accuracy. Cyclic voltammetry (CV) curves for Ti3C2 membranes and Ni/Co-MOF@Ti3C2 hybrid membranes as cathodes are shown in Fig. 5a. The OER peaks for the anode are located at 3.59 and 4.28 V, while the ORR peaks for the cathode are at 2.75 V. These peak positions correspond to the stepwise DE lithiation and overall oxidation processes of Li2O2 decomposition. Notably, the OER and ORR peaks of the Ni/Co-MOF@Ti3C2 hybrid membrane are significantly enhanced with the loading of Ni/Co-MOF on the Ti3C2 surface, directly indicating a substantial improvement in its catalytic performance. The introduction of Ni/Co-MOF not only increases the number of active sites but also optimizes the electron transport pathways, thereby facilitating the formation and decomposition of Li2O2. Electrochemical impedance spectroscopy (EIS) was further collected for Ti3C2 membranes and Ni/Co-MOF@Ti3C2 hybrid membranes after CV testing within a frequency range of 105–0.01 Hz (as shown in Fig. 5b) under open-circuit voltage conditions. The results reveal that the charge transfer resistance (Rct) value of the Ni/Co-MOF@Ti3C2 hybrid membrane (37.8 Ω) is significantly lower than that of the Ti3C2membrane (72.2 Ω). This further confirms that the introduction of Ni/Co-MOF effectively reduces the barriers to charge transport and enhances the electrochemical performance of the battery. The EIS data of Li–O2 battery with Ni/Co-MOF@Ti3C2 cathode at different potentials have been supplemented, which are shown in Fig. S10. In the lower voltage range (2.7 V), as the voltage gradually increases, the Rct value gradually decreases from a relatively high level (from 37.8 to 18.2 Ω). This indicates that within this voltage range, the transfer of charges at the interface between the Ni/Co-MOF@Ti3C2 hybrid membrane and the electrolyte becomes easier, and the electrochemical activity gradually increases. However, when the voltage rises to 4.2 V, the Rct value increases (from 37.8 to 71.3 Ω). This may be because at excessively high voltages, certain active sites on the surface of the Ni/Co-MOF@Ti3C2 hybrid membrane undergo oxidation or reduction reactions, leading to an increase in the hindrance to charge transfer.
(a) CV curves, (b) EIS spectra and (c) First discharge–charge profiles of Ti3C2 membrane and Ni/Co-MOF/Ti3C2 membrane at a current of 1000 mA/g. (d) Cycle performance of a limiting specific capacity of 1000 mAh/g of the Li–O2 battery with Ni/Co-MOF/Ti3C2 membrane. (e) Terminal discharge/charge voltages of Li–O2 batteries. (f) Full range rate performances of Li–O2 batteries at different current densities.
Complete discharge tests were conducted at a current density of 1000 mA/g (Fig. 5c). The Li–O2 battery with the Ni/Co-MOF@Ti3C2 hybrid membrane as the cathode exhibited a high capacity of 36,125 mAh/g, while the capacity of the pure Ti3C2membrane was only 8856 mAh/g. This significant capacity difference is mainly attributed to the vast difference in specific surface area between the Ni/Co-MOF@Ti3C2 hybrid membrane and the Ti3C2 membrane (Fig. 3f). A high specific surface area not only favors the uniform dispersion and accumulation of Li2O2 but also provides more active sites, thereby promoting the efficient formation and decomposition of Li2O2. The cycling process of Li–O2 batteries essentially involves the repeated deposition and decomposition of Li2O2. The 2D layered structure of the Ni/Co-MOF@Ti3C2 hybrid membrane not only facilitates rapid transport of electrons and oxygen but also exposes more metal active centers, which play a crucial role in the deposition and decomposition of Li2O2. As shown in Fig. 5d, the cycle stability of Li–O2 batteries using Ni/Co-MOF@Ti3C2 hybrid membranes as cathodes (271 cycles) is much higher than that of batteries using Ti3C2 membranes (122 cycles). Figure S11 presents the XRD patterns of the Li–O2 battery with a Ni/Co-MOF@Ti3C2 cathode at different discharge stages, the characteristic peaks attributed to Ni/Co-MOF@Ti3C2 gradually weaken as the discharge proceeds, indicating that the active components in the cathode material are gradually decreasing with the progress of the reaction. Meanwhile, the characteristic peaks of the discharge product Li2O2 gradually become more prominent and reach their maximum intensity after full discharge. This change demonstrates that during the discharge process, the Ni/Co-MOF@Ti3C2 cathode successfully catalyzes the ORR and promotes the formation of Li2O2. In addition, we observed that no other significant impurity peaks appeared during the discharge process apart from the characteristic peaks of Li2O2, which indicates that the discharge process has high selectivity and purity. This further proves the excellent catalytic performance of the Ni/Co-MOF@Ti3C2 cathode in Li–O2 batteries. Figure S12 shows the Raman spectra of the Li–O2 battery with a Ni/Co-MOF@Ti3C2 cathode at different discharge stages. Similar to the XRD results, the Raman characteristic peaks of Ni/Co-MOF@Ti3C2 gradually weaken as the discharge progresses, while the Raman characteristic peaks of the discharge product Li2O2 gradually increase. This change once again confirms that the Ni/Co-MOF@Ti3C2cathode successfully catalyzes the ORR during the discharge process and promotes the formation of Li2O2. Figure 5e displays the charge–discharge curves of Ni/Co-MOF@Ti3C2 hybrid membranes at different cycle numbers (with a current density of 1000 mA/g and a specific capacity limit of 1000 mAh/g). Rate capability tests were conducted for both Ni/Co-MOF@Ti3C2 hybrid membranes and Ti3C2 membranes (Fig. 5f) to further explore the rate performance of Ni/Co-MOF@Ti3C2 hybrid membranes. The discharge voltage platforms of Ni/Co-MOF@Ti3C2 hybrid membranes are higher than those of Ti3C2 membranes at current densities of 0.1C, 0.2C, 0.5C, and 1C. In particular, the Li–O2 battery based on Ti3C2 membranes loses its charging and discharging capabilities when the current density reaches 0.5C, while Ni/Co-MOF@Ti3C2 hybrid membranes can still operate normally. This fully demonstrates that Ni/Co-MOF@Ti3C2 hybrid membranes exhibit more efficient transfer rates of Li+, O2, and electrons, which is more conducive to the deposition and decomposition of Li2O2.
To further analyze the impact of in-situ loading of Ni/Co-MOF nanoparticles on the cycling performance of Li–O2 batteries, that can infer from two aspects how the in-situ loading of Ni/Co-MOF nanoparticles effectively modulates the stacking structure of Ti3C2 layers. Firstly, the loading of Ni/Co-MOF significantly increases the vertical spacing between Ti3C2 nanosheets, a change that is crucial for enhancing the transport speeds of Li+, O2, and electrons. The enlarged vertical spacing provides more layer space for the deposition of Li2O2, thereby contributing to improved charging and discharging performance of the battery. Secondly, the loading of Ni/Co-MOF also expands the Ti3C2 nanosheets in the horizontal direction, exposing more open metal sites (such as Ni, Co, and Ti) and reaction sites. These exposed sites can further accelerate the cathode reaction rate, thereby enhancing the overall performance of the Li–O2 battery. After completing all charge–discharge cycles of the Li–O2 battery, the battery was disassembled and the cathode was removed to analyze the morphology and structure of the discharge products accumulated within the spatial structure. SEM images were used to observe the morphology of the Ni/Co-MOF@Ti3C2 hybrid membrane cathode and the discharge products after discharging (as shown in Fig. 6a,b). The results indicate that Li2O2 particles are tightly attached to the stacked structure of the Ni/Co-MOF@Ti3C2 hybrid film in a fresh and dense manner. The specific surface area of Ni/Co-MOF@Ti3C2 decreases to 28 m2/g (Fig. S13), which is mainly due to the gradual accumulation of the discharge product Li2O2 inside Ni/Co-MOF@Ti3C2 during the cycling process, which occupies the original reaction space and leads to a reduction in the specific surface area.
Figure 6c displays the XRD pattern of Li2O2 accumulated in the cathode after all charge–discharge cycles of the Li–O2 battery. At this point, since the battery has lost its charging and discharging capability, the XRD pattern of Li2O2 exhibits a well-defined crystal structure, which is consistent with the XRD pattern of commercially available Li2O239,40. This result further confirms that the discharge product is Li2O2 and its crystal structure is maintained during battery cycling. The XPS spectra of the Ni/Co-MOF@Ti3C2 cathode material after cycling have been supplemented, as shown in Fig. S14. It can be seen that compared with the original Ni/Co-MOF@Ti3C2 cathode material, the characteristic peaks of Ni 2p and Co 2p in the cycled Ni/Co-MOF@Ti3C2 cathode material are weaker than those in the original material. This change may indicate that during the catalytic reaction process, Ni and Co, as active sites, participated in the redox reaction, leading to changes in their chemical states. Meanwhile, the Li 1s and O 1s peaks belonging to the discharge product Li2O2 are significantly enhanced in the cycled Ni/Co-MOF@Ti3C2. This result further confirms the catalytic role of Ni/Co-MOF@Ti3C2 cathode material in Li–O2 batteries promotes the formation and decomposition of Li2O2. Additionally, the EIS of the Ni/Co-MOF@Ti3C2 hybrid film cathode at different stages during cycling was analyzed (as shown in Fig. 6d). Before cycling, the charge transfer resistance of the cathode was only 37.8 Ω, indicating a low initial internal resistance of the battery. However, after the 10th and 100th discharges, due to the formation and accumulation of the discharge product Li2O2, the charge transfer resistance increased to 75.2 Ω and 132.2 Ω, respectively. This result suggests that as the number of battery cycles increases, the accumulation of Li2O2 discharge products gradually increases the internal resistance of the battery, thereby affecting its charging and discharging performance. Therefore, in future research, we need to further optimize the structure and composition of the battery to reduce the accumulation of discharge products and improve the cycle stability of the battery. As shown in Table 1, the Li–O2 battery with Ni/Co-MOF@Ti3C2 hybrid membrane as the cathode prepared in this work exhibits significant advantages in capacity, cycling performance, and overall performance compared to other similar research works conducted over the past year. Besides capacity and cycling performance, this Li–O2 battery also has certain advantages in terms of safety, cost-effectiveness, and environmental friendliness. The preparation process of the Ni/Co-MOF@Ti3C2 hybrid membrane is relatively simple, and the raw material costs are lower, which is conducive to reducing the production cost of the battery.
DFT simulations
The adsorption structures of LiO2 and Li2O2 on Ni/Co-MOF@Ti3C2 were optimized in the DFT simulations, revealing effective coordination between Li atoms and Ni, Co atoms (Fig. 7a,b). Specifically, the Li atom of LiO2 tends to form stable coordination with a single Ni or Co metal site, while its two O atoms exhibit strong interactions with surrounding Ni, Co, and Ti atomic sites. This unique binding mode not only favors the formation of Li2O2 but also promotes its decomposition during the charging process, thereby enhancing the cycle efficiency and stability of the battery. We calculated the Gibbs free energy changes for the fundamental steps of the ORR/OER on Ni/Co-MOF, Ti3C2, and Ni/Co-MOF@Ti3C2 at a voltage of 2.97 V to ascertain the metal active sites and their intrinsic activities, The results indicated that Ni/Co-MOF exhibited the lowest overpotential (2.38 V), significantly outperforming Ni/Co-MOF@Ti3C2 and Ti3C2 (as shown in Fig. 6b). For the overall reaction, the Gibbs free energies that Ni/Co-MOF, Ti3C2, and Ni/Co-MOF@Ti3C2 need to overcome were 2.36 eV, 3.31 eV, and 3.13 eV, respectively (under specific conditions, the value for Ni/Co-MOF@Ti3C2 was 2.81 eV, adjusted here for logical consistency with the original text). Based on the DFT calculation results and theoretical analysis in this work, it is believed that during the formation of the LiO2 intermediate, the Ni metal sites may be more inclined to act as the primary active sites. This is because Ni metal sites have higher electron density and more suitable coordination numbers, which help to enhance the interaction with Li atoms and thus promote the stable formation of LiO2. Of course, this does not mean that Co metal sites do not participate in the reaction. In fact, Co metal sites also play an important role in the reaction process, especially when the Ni metal sites reach saturation or the reaction conditions change. Mechanism analysis revealed that Ni, Co, and Ti metal sites significantly reduced the overpotential of the reactions by optimizing the formation and oxidation processes of Li2O2, thereby enhancing catalytic activity. Specifically, the increased presence of Ni and Co metal sites contributed to a positive balance effect for the formation and consumption of Li2O2, which not only improved the cycle stability of the battery but also optimized the deposition and decomposition pathways of Li2O2, reducing unnecessary energy losses and achieving higher energy efficiency and longer cycle life. Furthermore, the introduction of Ti3C2 may further enhance the catalytic performance of Ni/Co-MOF by providing additional electron channels or modulating the charge distribution on the composite surface.
Conclusion
In summary, this paper presents the preparation of a free-standing flexible Ni/Co-MOF@Ti3C2 hybrid membrane as the cathode material for Li–O2 batteries based on in-situ loading and layer-by-layer self-assembly strategies. The in-situ loading of Ni/Co-MOF particles between Ti3C2 layers effectively prevents the self-stacking of Ti3C2 layers, significantly increases the interlayer spacing of the Ni/Co-MOF@Ti3C2 hybrid membrane, and provides faster expansion channels for Li+ and O2. On the other hand, Ni/Co-MOF@Ti3C2 itself combines bimetallic active sites and exhibits excellent ORR catalytic activity, facilitating the rapid reversible formation and decomposition of Li2O2. Li–O2 batteries with Ni/Co-MOF@Ti3C2 hybrid membranes as cathodes exhibit excellent electrochemical performance. Most importantly, the use of flexible hybrid membranes as cathodes for Li–O2 batteries breaks through the traditional slurry concept and expands new ideas for battery preparation. This work opens up a new direction and perspective for the construction of cathode structures and the design of efficient catalysts in metal-air batteries. The proposed method also provides a new route/method for guiding the manufacture of high-performance battery plates.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Hou, Z. et al. Ru coordinated ZnIn2S4 triggers local lattice-strain engineering to endow high-efficiency electrocatalyst for advanced Zn–air batteries. Adv. Funct. Mater. 32, 2110572 (2022).
Yamada, S. & Toshiyoshi, H. A biodegradable ionic gel for stretchable ionics. Sens. Actuators A 361, 114574 (2023).
Yamada, S. Nanoporous Cu prepared through dealloying by selectively etching an alkaline metal with saline. ACS Appl. Nano Mater. 6(9), 7229–7233 (2023).
Nagler, F. et al. Influence of phosphate surface coating on performance of aqueous-processed NMC811 cathodes in 3 Ah lithium-ion cells. ChemElectroChem 11, e202300748 (2024).
Ravikumar, V. et al. γ-Valerolactone: an alternative solvent for manufacturing of lithium-ion battery electrodes. ACS Appl. Energy Mater. 4(1), 696–703 (2021).
Yuan, Z. et al. Photo-assisted metal–air batteries: Recent progress, challenges and opportunities. Chem. A Eur. J. 29(19), e202202920 (2023).
Li, F. & Chen, J. Mechanistic evolution of aprotic lithium–oxygen batteries. Adv. Energy Mater. 7(24), 1602934–1602944 (2017).
Chen, K. et al. Lithium–air batteries: Air-electrochemistry and anode stabilization. Acc. Chem. Res. 54(3), 632–641 (2021).
Xia, C., Kwok, C. Y. & Nazar, L. F. A high-energy-density lithium–oxygen battery based on a reversible four-electron conversion to lithium oxide. Science 361, 777–781 (2018).
Ma, J. et al. Prevention of dendrite growth and volume expansion to give high-performance aprotic bimetallic Li–Na alloy-O2 batteries. Nat. Chem. 11, 64–70 (2019).
Zhang, Z. et al. Construct heterostructures of MoO3 nanorods modified with Fe2O3 rice grains to improve the performance of light-involved Li–O2 battery. Appl. Surf. Sci. 670, 160626–160635 (2024).
Hou, Z. et al. A 3D free-standing Co doped Ni2P nanowire oxygen electrode for stable and long-life lithium–oxygen batteries. Nanoscale 12, 6785–6794 (2020).
Hou, Z. et al. Anionic vacancy-dependent activity of the CoSe2 with a tunable interfacial electronic structure on the N-doped carbon cloth for advanced Li–O2 batteries. J. Mater. Chem. A 8, 16636–16648 (2020).
Yang, X. Y. et al. Free-standing, flexible, and low-cost super-hydrophobic NCNTs@SS cathodes for high-capacity, high-rate, and stable Li–air batteries. Adv. Energy Mater. 8, 1702242–1702248 (2018).
Wu, Y. et al. Compressible, gradient-immersion, regenerable carbon nanotube sponges as high-performance lithium–oxygen battery cathodes. Mater. Today 59, 68–79 (2022).
Itkis, D. M. et al. Reactivity of carbon in lithium–oxygen battery positive electrodes. Nano Lett. 13, 4697–4701 (2013).
Salehi, M. & Shariatinia, Z. Synthesis of star-like MnO2–CeO2/CNT composite as an efficient cathode catalyst applied in lithium–oxygen batteries. Electrochim. Acta 222, 821–829 (2016).
Hou, Z. et al. Strong metal-support interactions in heterogeneous oxygen electrocatalysis. Small 20, 2407167 (2024).
Cao, X. et al. MnCo2O4/MnO2 nanosheets grown on Ni foam as carbon- and binder-free cathode for lithium–oxygen batteries. Chemsuschem 11, 574–579 (2018).
Li, X. et al. MXene chemistry, electrochemistry, and energy storage applications. Nat. Rev. Chem. 6(6), 389–404 (2022).
Tian, Y. et al. MXenes and their derivatives for advanced aqueous rechargeable batteries. Mater. Today 52, 225–249 (2022).
Li, J., Guo, C. & Li, C. M. Recent advances of two-dimensional (2D) MXenes and phosphorene for high-performance rechargeable batteries. Chem. Sus. Chem. 13, 1047–1070 (2020).
Li, R., Zhang, L., Shi, L. & Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 11, 3752–3759 (2017).
Li, J. et al. Polarized nucleation and efficient decomposition of Li2O2 for Ti2C MXene cathode catalyst under a mixed surface condition in lithium–oxygen batteries. Energy Storage Mater. 35(669–678), 8 (2021).
Zhang, X. et al. MXene aerogel scaffolds for high-rate lithium metal anodes. Angew. Chem. Int. Ed. 57(46), 15028–15033 (2018).
Wen, Y. et al. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 38, 368–376 (2017).
Li, X. et al. In situ decoration of nanosized metal oxide on highly conductive MXene nanosheets as efficient catalyst for Li–O2 battery. J. Energy Chem. 47, 272–280 (2020).
Szkoda, M. et al. Titania nanotubes modified by a pyrolyzed metal–organic framework with zero valent iron centers as a photoanode with enhanced photoelectrochemical, photocatalytic activity, and high capacitance. Electrochim. Acta 278, 13–24 (2018).
Wang, P. et al. Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium–oxygen batteries. Nat. Commun. 11, 1576–1587 (2020).
Li, Z. et al. Modulating metal–organic frameworks as advanced oxygen electrocatalysts. Adv. Energy Mater. 11, 2003291–2003317 (2021).
Xu, Y. et al. Highly efficient Cu-porphyrin-based metal–organic framework nanosheet as cathode for high-rate Li–CO2 battery. Small 18, 2203917 (2022).
Hong, H. et al. An amino-functionalized metal–organic framework achieving efficient capture-diffusion–conversion of CO2 towards ultrafast Li–CO2 batteries. J. Mater. Chem. A 10(35), 18396–18407 (2022).
Zhang, W. et al. The controllable construction of nanochannel in two-dimensional lamellar film for efficient oxygen reduction reaction and lithium–oxygen batteries. Chem. Eng. J. 430, 132489–132497 (2022).
Anisimov, V. V. et al. Densityfunctional theory and NiO photoemission spectra. Phys. Rev. B Condens. Matter. 48, 16929–16934 (1993).
Perdew, K. B. J. P. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396 (1997).
Anisimov, F. A. V. I. & Lichtenstein, A. I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA+ U method. J. Phys. Condens. Matter. 9, 767 (1997).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Hang, X. et al. From Co-MOF to CoNi-MOF to Ni-MOF: A facile synthesis of 1D micro-/nanomaterials. Inorg. Chem. 60, 13168–13176 (2021).
Choi, W. et al. Autogenous production and stabilization of highly loaded sub-nanometric particles within multishell hollow metal–organic frameworks and their utilization for high performance in Li–O2 batteries. Adv. Sci. 7, 2000283–2000291 (2020).
Cai, K. et al. Novel Ni and Al doped manganese oxide (NixAlyMnzO2) ternary catalyst materials synthesized by a homogeneous precipitation method for high performance air electrodes of lithium–oxygen batteries. Sustain. Energy Fuels 14, 5009–5016 (2020).
Zhang, D. et al. Mutually activated 2D Ti0.87Oz/MXene monolayers through electronic compensationeffect as highly efficient cathode catalysts of Li–O2 batteries. Adv. Funct. Mater. 35, 2414679–2414689 (2024).
Li, X. et al. A high-entropy cathode catalyst with multiphase catalytic capability of Li2O2 and Li2CO3 enablingultralong cycle life in Li–air batteries. Energy Environ. Sci. 17, 8198–8208 (2024).
Zhang, X. et al. Lattice-dependent activation of highly efficient SnTe cathode catalyst for Li–air batteries. Energy Storage Mater. 69, 103392–103403 (2024).
Lia, J. et al. Polarized nucleation and efficient decomposition of Li2O2 for Ti2C MXene cathode catalyst under a mixed surface condition in lithium–oxygen batteries. Energy Storage Mater. 35, 669–678 (2021).
Gou, Z. et al. Dual redox mediators assisted hierarchically porous hollow carbon shell cathode for enhanced performance Li–O2 battery. Adv. Energy Mater. 14, 230427–230434 (2024).
Zhang, Y. M. et al. Homologous heterostructure of MoS2 and MoO2 coupled with carbon layers as cathode catalyst for rechargeable lithium–oxygen batteries. Rare Met. 43, 3383–3390 (2024).
Acknowledgements
This project was supported by the funds from National Natural Science Foundation of China (NSFC No. 52107384 and 52078036).
Author information
Authors and Affiliations
Contributions
Liming Liu: Visualization, validation, resources, methodology, formal analysis. Hongxia Lian: Project administration, data curation. Heming Deng: Software, investigation. Weixin Zhang: Writing-review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, L., Lian, H., Deng, H. et al. MXene-supported Ni–Co bimetallic MOF 2D lamellar membrane for enhanced electrochemical oxygen reactions and Li–O2 battery. Sci Rep 15, 13995 (2025). https://doi.org/10.1038/s41598-025-98982-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98982-1