Abstract
Τhe effects of recombinant meagre follicle stimulating hormone (rFsh) and luteinizing hormone (rLh) were evaluated on testicular maturation in pre-pubertal meagre Argyrosomus regius, an upcoming aquaculture species in the Mediterranean region. Fish (body mass 1092.9 ± 178.8 g) received seven weekly injections of meagre rFsh, together with an rLh injection at week 6. One group was further treated with weekly rFsh or rLh until week 11 and was sacrificed at week 12 (rFsh/rLh group, TREATED 12). The second group received further weekly injections of rFsh until week 12 and was then left untreated until week 21 (single rLh group, TREATED 21). Control fish received injections of saline solution. At week 12, the testes of the rFsh/rLh-treated fish (TREATED 12) showed increased gonadosomatic index and seminiferous tubule diameter, reduction of spermatogonial density, increase of post-meiotic spermatocysts and accumulation of luminal spermatozoa. In the single rLh-treated group (TREATED 21), apoptosis increased towards pre-treatment levels, demonstrating that the withdrawal of the Fsh stimulus ceased the process of spermatogenesis. The results demonstrated the effectiveness of rFsh/rLh treatment in stimulating testicular growth, spermiogenesis and spermiation in pre-pubertal meagre.
Similar content being viewed by others
Introduction
The meagre Argyrosomus regius (Asso, 1801) is a member of the Sciaenidae family with a wide distribution in coastal waters, including brackish estuaries and lagoons of the eastern Atlantic Ocean and Mediterranean Sea1. This species is a recently established aquaculture species with a gradually increasing production in Mediterranean countries and very high production in Egypt2,3. As widely documented in many fish species4,5,6,7,8,9,10,11,12,13,14, meagre broodstocks reared in captivity fail to spawn spontaneously and hormonal treatments have been developed to stimulate oocyte maturation and ovulation, enhance sperm production and induce spawning of eggs with high fertilization success15,16,17,18,19,20,21,22,23. It was recently reported in a commercial farm in the North Ionian Sea (Europe), that rearing in sea cages may reduce captivity-induced stress and result in spontaneous maturation and spawning, since females had ovaries with post-ovulatory follicles during the reproductive season, an indication of recent spawning24.
Puberty in vertebrates involves the activation of the reproductive axis, a process involving the release of follicle stimulating hormone (Fsh) and luteinizing hormone (Lh) from the pituitary, which in turn induce gonad development and gametogenesis through the stimulation of sex steroid hormone synthesis and secretion25,26. It is well known that Fsh plays a major regulatory role during early stages of spermatogenesis, stimulating spermatogonia proliferation, while Lh is mainly involved in the final stages of maturation, the spermiogenesis27. At the completion of puberty, the previously juvenile individual is now a reproductively mature one, and in regards to the males, the testes produce spermatozoa that can be released to the environment during spawning. Lowering the age at puberty in aquaculture will reduce generation time in selective breeding programs, speed up breeding gains of desirable traits (growth, feed efficiency, disease resistance, etc.) and improve the cost of broodstock maintenance, by reducing the time required for fish to become reproductively active. Therefore, several studies have reported efforts to induce puberty precociously in fish, using gonadotropin releasing hormone agonists (GnRHa), gonadotropins (Gths) and sex steroid hormones, but the results have been variable28,29,30,31,32,33,34. In recent years, recombinant gonadotropins (rGths) have proven to be powerful tools to alleviate fish reproductive dysfunctions and recombinant Fsh (rFsh) has been used successfully to enhance spermatogenesis in greater amberjack Seriola dumerili35 Senegalese sole Solea senegalensis36,37, European eel Anguilla anguilla38,39 and flathead grey mullet Mugil cephalus40,41.
In a previous study42, a six-week rFsh treatment was found to be effective in reducing germ cell death by apoptosis in 18 month-old pre-pubertal meagre that showed signs of spermatogenesis activation, but had not yet reached sexual maturity. Following these encouraging results, we report here the effects of the co-administration of rFsh and rLh on testicular development and reproductive maturation of pre-pubertal meagre, as well as the effects of rGth withdrawal on spermatogenesis.
Materials and methods
Ethics
The study was in accordance with the European Directive, the Spanish Royal Decree and the Catalan Law for the protection of animals used for scientific purposes. The present study was approved by IRTA’s (Institute of Agrifood Research and Technology) Committee of Ethics and Experimental Animal (CEEA) and the Catalan Government as experimental project 11,264 with expedient number FUE-2020-01809522 and ID CJQX0B0PH. The authors complied with the ARRIVE guidelines.
Production of recombinant gonadotropins
The α- and β-subunit amino acid sequences of the Gths were deduced from the meagre pituitary mRNA sequences deposited in the European Nucleotide Archive (ENA) under the project accession numbers PRJEB57583. The deduced α subunit sequence common to both gonadotropins and the Fsh β sequence have been reported by Zupa et al.42.
The deduced Lh β subunit sequence is:
Single-chain meagre rFsh and rLh were produced by Rara Avis Biotec S.L (Valencia, Spain) following the procedure described for the Senegalese sole and already reported in Zupa et al. (2023)42 for rFsh. Briefly, Chinese hamster ovary (CHO-S) cells in suspension were transfected with an expression construct encoding a fusion protein containing the entire coding sequence of meagre Fsh β or rLh β subunit, the 28 amino acids of the carboxyl-terminal sequence of the human corionic gonadotropin (hCG β) β subunit as a linker, and the mature sequence of meagre α subunit (Cga). After 120 h of CHO cell culture, ion exchange chromatography was used to purify the secreted recombinant hormones from the culture medium. The hormones were concentrated to 12 µg mL− 1 and quantified by semiquantitative Western blot, using polyclonal (mouse) antibody against meagre Fsh β or rLh β subunit and metal affinity purified His-tagged meagre rFsh and rLh as standard.
Fish rearing and administration of recombinant gonadotropins
Juvenile meagre used for the present study were produced in IPMA (Olhão, Portugal) hatchery in the spring of 2020, and then transferred to IRTA during November 2020, where they were reared in a 10 m3 tank under natural photoperiod and constant temperature (18.1 ± 0.3 °C). At the starting date of the experiment (27 October 2021), the fish had mean body mass of 1.08 ± 0.25 kg and a total length of 43.6 ± 3.8 cm. During the experiment, fish were fed daily, 6 days a week to apparent satiation with pellets (Brood Feed Lean, SPAROS, Portugal), and were starved for 24 h before hormone injections and samplings. For all the hormone injection and sampling, fish were anaesthetized with 70 mg L− 1 of MS-222.
The fish were divided in three groups, a control group and two rGth-treated groups. Both rGth-treated groups received weekly injections of rFsh starting at week 0 and for six consecutive weeks as reported by Zupa et al. (2023)42, and received the first rLh injection on week 6 (Fig. 1). Then, one group was treated further with rFsh for three more weeks (week 7, 8 and 9) and received again rLh injections on weeks 10 and 11; these fish were sacrificed and sampled on week 12 (rFsh/rLh group, TREATED 12 group; N = 8). This protocol was aimed to maximize both spermatogenesis and spermiation over a 12-week treatment period. The other rFsh/rLh-treated group continued to receive weekly injections of rFsh for six more weeks and then was left untreated; these fish were sacrificed and sampled on week 21 (single rLh group, TREATED 21 group; N = 6). This protocol was aimed to assess the effects of the rFsh withdrawal after a long-term treatment, on spermatogenesis, spermatogonial proliferation and germ cell apoptosis. The control group was given a weekly 1 mL injection of saline solution from the beginning of the experiment and individuals were sacrificed at the beginning of the experiment (CONTROL 0 group; N = 5), on then at week 12 (CONTROL 12 group; N = 5) and week 21 (CONTROL 21 group; N = 3), at the times the two rFsh/rLh-treated groups were sacrificed.
Experimental design of treatment with rFsh and rLh showing the doses of rFhs and rLh used. Doses of rFsh and rLh are highlighted in light blue and cream yellow, respectively. Then, treated fish were divided in two groups that were treated as reported in the diagram. Fish from TREATED 12 group were sacrificed and sampled on week 12 and fish from TREATED 21 group were sacrificed and sampled on week 21. Red boxes indicate the sampling week. Control fish were sacrificed at week 0, 12 and 21.
Fish sampling
In order to analyse the effects of the treatments on testicular maturation, fish were sacrificed at planned dates with an overdose of anesthesia (MS-222) followed by pithing to destroy the brain. Fish total length (TL, in cm), body mass (BM, in g) and gonad mass (GM, in g) were measured and gonadosomatic index (GSI) was calculated as 100 x GM/BM. Moreover, one-cm thick gonad slices were cut and fixed for four hours in Bouin’s solution, and then stored in 70% ethanol.
Testis histology, immunohistochemistry and identification of apoptotic germ cells
Testis samples were dehydrated in ethanol, clarified in xylene and embedded in paraffin wax. Four-µm thick sections were cut and stained with haematoxylin-eosin (H-E) or destined to immunohistochemical analysis and to the detection of apoptotic germ cells.
Proliferating germ cells were identified through the immunohistochemical localization of the Proliferating Cell Nuclear Antigen (PCNA), a polymerase delta accessory protein used as marker of proliferation, according to the procedure described in Zupa et al. (2013, 2017)6,9. Briefly, endogenous peroxidase was inhibited by pre-treating sections for 10 min with 3% H2O2. Subsequently, sections were incubated for 30 min in normal horse serum (NHS; Vector, Burlingame, Ca) and then incubated overnight in a moist chamber at 4 °C with monoclonal antibodies to PCNA (Santa Cruz Biotechnology Inc., Dallas, Texas) diluted 1:25 in phosphate buffered saline (PBS; 0.01 M, pH 7.4, containing 0.15 M NaCl) containing 0.1% bovine serum albumin (BSA; Sigma-Aldrich, Milan, Italy). The visualization of the immunohistochemical reaction was performed through the avidin-biotin-peroxidase complex (ABC) procedure using the Vectastain Universal Elite Kit (Vector, Burlingame, Ca). Peroxidase activity was visualized by incubating for 10 min with a 3,3′-diaminobenzidine (DAB) Peroxidase Substrate Kit (Vector, Burlingame, Ca). Replacement of the primary antibody with NHS and PBS was used as control procedure to confirm the immunoreaction specificity.
The identification of apoptotic germ cells was carried out through the terminal deoxynucleotidyl transferase-mediated d’UTP nick end labeling (TUNEL) method with an in situ Cell Death Detection Kit, AP (Roche Diagnostics, Mannheim, Germany)6,9. Incubation with the reaction mixture was preceded by treating sections with a permeabilization solution of 0.1% Triton X-100 in 0.1% sodium citrate for 8 min. Terminal deoxynucleotidyl transferase was diluted 1:2 in TUNEL Dilution Buffer (Roche Diagnostics, Mannheim, Germany), and a ready-to- use solution of nitro-blue tetrazolium chloride/5-bromo-4-chloro-3’-indolyphosphate p-toluidine salt (NBT/BCIP) (Roche Diagnostics, Mannheim, Germany) served as a substrate for the signal conversion.
Relative quantification of germ cell types
The different germ cell types were identified based on their size and morphology as described by Zupa et al.43. Briefly, single undifferentiated type A (Aund) spermatogonia were the largest germ cells (mean diameter 15.5 ± 1.8 μm); spermatogonia committed towards spermatogenesis (differentiated type A, Adiff and type B) were smaller cells contained in spermatocysts of two or more cells; spermatocytes showed a variable morphology according to the different phases of meiosis, and spermatids had a compact and strongly basophilic nucleus (Fig. 2).
The density of single type Aund spermatogonia (n cells/mm2 germinal epithelium), and the relative surface occupied by committed spermatogonia (type Adiff + type B), spermatocytes, and spermatids (germ cell surface/mm2 of germinal epithelium) were measured in five randomly-selected digital fields from the peripheral (proliferative) testis region. All the above measurements were taken from microphotographs captured with a digital camera (K3, Leica, Wetzlar, Germany) connected to a light microscope (DMRB, Leica, Wetzlar, Germany), using image-analysis software (LAS X, Wetzlar, Germany).
Seminiferous tubule diameter, proliferation index and quantification of testicular apoptosis
The diameter of seminiferous tubules was determined on at least 80 tubules randomly selected in the peripheral region of testis sections stained with H-E sections. The proliferation (mitotic) index was calculated as percentage of PCNA-positive single type Aund spermatogonia. To this aim, PCNA positive and negative type Aund spermatogonia were counted on at least five randomly selected digital fields of the proliferative (peripheral) testis region. Due to the fragmentation of apoptotic cells into ‘apoptotic bodies’44 and the loss of the nuclear morphology in TUNEL-stained sections, which made individual identification of cells involved in the apoptotic process difficult, apoptosis was quantified by measuring the surface area occupied by TUNEL-positive apoptotic structures (µm2 per mm− 2 testis tissue). All the above measurements were performed on digital fields using the same image analysis system described above.
Statistical analysis
Statistical differences in GSI, density of single type Aund spermatogonia, surface occupied by spermatogonial, spermatocyte, and spermatid cysts, seminiferous tubule diameter, proliferation index, as well as surface occupied by apoptotic structures were evaluated by an ANOVA followed by Tukey-Kramer post hoc test. Prior to the ANOVA, normality of variance was assessed through Shapiro-Wilk W test and percentage and proportion data were arcsine transformed45. Statistical analyses were performed with SAS® OnDemand for Academics (SAS Institute Inc., Cary, NC, USA). The results are presented as means ± sd, with statistical probability significance established at the P < 0.05 level.
Results
Biometric data and gonadosomatic index, seminiferous tubule diameter, testis histology and relative quantification of germ cell types
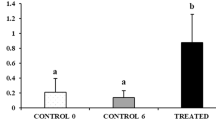
Biometric data (FL, BM and GM) of fish sampled for the present study are reported in Table 1. The GSI did not change significantly over time among the three control groups (CONTROL 0 vs. CONTROL 12, P = 0.99; CONTROL 12 vs. CONTROL 21, P = 0.77) or between the two treated groups (P = 0.99); the TREATED 12 and TREATED 21 groups had significantly higher GSI compared with their respective Controls (P < 0.05 in both cases) (Fig. 3a). The diameter of seminiferous tubules showed the same trend of GSI: it did not change significantly among the three control groups (CONTROL 0 vs. CONTROL 12, P = 0.99; CONTROL 12 vs. CONTROL 21, P = 0.98) or between the two treated groups (P = 0.99), but it was significantly higher in TREATED 12 and TREATED 21 groups compared with their respective Controls (P < 0.05 in both cases) (Fig. 3b).
Fish from the CONTROL 0 group had small testes with seminiferous tubules with no or a very small lumen and a small number of spermatozoa in the main sperm duct (Fig. 4a). In proliferative (peripheral) region, mainly spermatogonia and spermatocytes were observed (Fig. 4b). The testes of the CONTROL 12 males showed seminiferous tubules with all stages of spermatogenesis, and a small number of luminal spermatozoa in seminiferous tubules and main sperm duct (Fig. 4c, d). Three out of eight fish of the TREATED 12 group were in active spermatogenesis, showing germ cells in all stages of spermatogenesis in the peripheral testis region and accumulation of large amounts of luminal spermatozoa in seminiferous tubules and sperm duct system (Fig. 4e, f). The other five fish of the TREATED 12 group and all the fish of the TREATED 21 (Fig. 4i, l) showed arrested spermatogenesis with seminiferous tubules devoid of spermatocysts; both the tubule lumina and sperm duct system were filled with spermatozoa. Spermatogenesis was regressed also in the testes of two fish of the CONTROL 21 group, as the seminiferous epithelium was almost devoid of spermatocysts and seminiferous tubules contained scarce luminal spermatozoa (Fig. 4g, h). One of the fish of this group had a higher GSI (2.2) and larger seminiferous tubules than the other fish of the same group, and its seminiferous epithelium showed an active spermatogenesis (Supplementary File S1, fish ID Exp2_M21_33).
Micrographs of testis sections from juvenile meagre. (a–b) CONTROL 0; (c–d) CONTROL 12; (e–f) TREATED 12; (g–h) CONTROL 21; (i–l) TREATED 21. H–E staining. Micrographs (b), (d), (f), (h), and (l) are higher magnifications of the area included in the red rectangles of (a), (c), (e), (g), and (i), respectively. Magnification bar = 1 mm in (a), (c), (e), (g), (i) and 300 μm in (b), (d), (f), (h), (l). sz, spermatozoa.
The quantitative analysis of the different germ cell types showed no statistical difference in the density of single type Aund spermatogonia among the three control groups (CONTROL 0 vs. CONTROL 12, P = 0.97; CONTROL 12 vs. CONTROL 21, P = 0.88) or between the two treated groups (P = 0.57); the density of these cells was significantly lower in the TREATED 12 group compared with its respective Control (P < 0.05), but did not differ between TREATED 21 and its respective Control (Fig. 5a). No statistical difference in the germinal epithelium surface occupied by cysts containing committed spermatogonia (Type Adiff and Type B) was observed among the control groups (CONTROL 0 vs. CONTROL 12, P = 0.93; CONTROL 12 vs. CONTROL 21, P = 0.53) or between the two treated groups (P = 0.97); the germinal epithelium surface occupied by committed spermatogonia was significantly lower in TREATED 12 and TREATED 21 groups compared to their respective Controls (P < 0.05 in both cases) (Fig. 5b). No statistical difference in the germinal epithelium surface occupied by spermatocyte cysts was observed among the control groups (CONTROL 0 vs. CONTROL 12, P = 0.28; CONTROL 12 vs. CONTROL 21, P = 0.21) and between each treated group and their respective Controls (TREATED 12 vs. CONTROL 12, P = 0.63; TREATED 21 vs. CONTROL 21, P = 0.89); a reduction of the density of spermatocyte cysts was observed in treated fish from week 12 to week 21 (TREATED 21 vs. TREATED 12, P < 0.05) (Fig. 5c). No statistical difference in the germinal epithelium surface occupied by spermatid cysts was observed among the control groups (CONTROL 0 vs. CONTROL 12, P = 0.89; CONTROL 12 vs. CONTROL 21, P = 0.83); the surface occupied by these cells was significantly higher in the TREATED 12 group compared with the respective Control (P < 0.05) and it was similar between TREATED 21 and CONTROL 21 groups (P = 1.0); a reduction of the density of spermatid cysts was observed in treated fish from week 12 to week 21 (TREATED 21 vs. TREATED 12, P < 0.05) (Fig. 5d).
Changes in mean (± sd) (a) density of single type Aund spermatogonia, and (b), (c), (d) surface occupied by spermatogonial or spermatocyte or spermatid cysts, respectively, in control or rGths-treated juvenile meagre. Different letters represent statistically significant differences (ANOVA; P < 0.05).
Proliferation index and apoptosis
The immunostaining with anti-PCNA antibodies labelled nuclei of single type Aund spermatogonia, committed spermatogonia and primary spermatocytes (Fig. 6).
Micrograph of testis sections of a juvenile meagre immunostained with antibodies against the Proliferating Cell Nuclear Antigen (PCNA), which stains brown the nuclei of proliferating cells. Magnification bar = 30 μm. Arrowhead = anti-PCNA positive single type Aund spermatogonium; dashed arrow = anti-PCNA negative single type Aund spermatogonium; curved arrow = anti-PCNA positive type B spermatogonia; arrow = anti-PCNA positive primary spermatocytes.
No statistical difference in the proliferation index of single type Aund spermatogonia was observed between control and treated fish; a significant decrease of the proliferation index was observed in both groups of fish sampled at week 21 compared with groups sampled at week 12 (P < 0.05 in both cases) (Fig. 7).
TUNEL-positive structures cells, likely Sertoli cells and spermatogonia, were observed in all the examined samples (Fig. 8). The surface occupied by apoptotic structures was significantly larger in the CONTROL 0 compared with the CONTROL 12 group (P < 0.05); no difference in the surface occupied by apoptotic structures was observed between CONTROL 0 and CONTROL 21 (P = 0.09), CONTROL 12 and TREATED 12 (P = 1.0) and CONTROL 21 and TREATED 21 groups (P = 0.18); the TREATED 21 group had significantly higher apoptotic structure density than the TREATED 12 group (P < 0.05) (Figs. 8 and 9).
Micrographs of testis sections of juvenile meagre stained with the terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick end labelling (TUNEL) method, with apoptotic structures appearing as dark blue dots. (a), CONTROL 0; (b), CONTROL 12; (c), TREATED 12; (d), CONTROL 21; (e), TREATED 21. (f) and (g) are higher magnifications of the area included in the red rectangles of (a). (f) shows scattered apoptotic structures in the peripheral (proliferative) testis region and diffuse apoptotic structures along the perimeter of seminiferous tubules of the intermediate testis region. (g) shows apoptotic Sertoli cells (arrowheads) and spermatogonia (arrows). (h) is a higher magnification of the area included in the red rectangles of (e), and shows an apoptotic spermatogonial cyst (arrow). Magnification bar = 1 mm in (a), (b), (c), (d), and (e); 200 μm in (f); 50 μm in (g); 100 μm in (h). p, peripheral region; i, intermediate region; c, central region.
Discussion
In meagre, as well as in many other fish affected by captivity-induced reproductive dysfunctions, treatment protocols based on GnRHa administration have been proved to be effective in stimulating oocyte maturation and ovulation, enhancing sperm production and inducing spawning15,16,17,18,19,20,21,22,23. These spawning-induction protocols are commonly applied to alleviate reproductive dysfunctions caused by an insufficient Lh release from the pituitary, but they are not effective in inducing pituitary Fsh synthesis or secretion46,47. Although administration of GnRHa or human chorionic gonadotropin (hGC), the latter acting as an Lh-like hormone, is not expected to be effective in inducing gametogenesis in immature fish, which requires both Fsh and Lh25,26, in female red seabream Pagrus major, GnRHa administration has been reported to be effective in inducing precocious puberty48, an effect that may be explained by the peculiarly negligible role of Fsh in the oogenesis of this species48.
The effectiveness of rFsh and rLh -synthesized using different methodologies- in stimulating the onset of spermatogenesis and testicular growth has been demonstrated widely in several fish species37,38,41. In meagre, it was recently reported that treatments with rFsh and rLh were not effective in inducing puberty in 9-month-old juveniles34, whereas rFsh triggered the onset of testis growth and spermatogenesis in 18-month-old pre-pubertal individuals42, i.e. individuals that showed signs of spermatogenesis activation but had not completed sexual maturation during the annual reproductive season, and did not produce mature, releasable spermatozoa. Pre-pubertal meagre treated with rFsh showed active spermatogenesis, an increase of proliferation of spermatogonia committed towards spermatogenesis as well as the entry of spermatogonia into meiosis42. The present study describes the effects on the latter phases of testicular maturation of the administration of a combination of rFsh/rLH, according to a protocol applied after the six-week rFsh treatment reported in42. The applied combination of rFsh/rLh stimulated further testicular growth, as evidenced by an increase in GSI that persisted 9 weeks after the end of the rFsh/rLh administration. The GSI of rFsh/rLh-treated fish (≈ 2%) was still lower compared with adult meagre reared commercially in sea cages (≈ 4%;24); however, testes of fish from both rFsh/rLh-treated groups had larger seminiferous tubules than controls, and slightly lower than that of adult meagre sampled in June at the peak of the reproductive season (≈ 160 μm vs. ≈ 200 μm). The treatment with rFsh/rLh induced further advancement of spermatogenesis and accumulation of large sperm masses in three of the eight individuals of the TREATED 12 group, and finalization of the spermatogenesis process in the other five individuals of the same group, and in all individuals of the TREATED 21 group that showed seminiferous tubules devoid of spermatocysts and filled with spermatozoa. Moreover, from only one out of 13 control fish (considered as a precociously maturing fish), produced sperm was collected when abdominal pressure was applied, whereas viable sperm (percentage motile spermatozoa 64.5 ± 16.0%; cell concentration 2.0 ± 1.1 × 1010 cells mL− 1; curvilinear velocity 103.7 ± 23 μm s− 1; straight-line velocity 72.5 ± 15.5 μm s− 1 and average path velocity 90.4 ± 20.3 μm s− 1) was obtained from all the treated fish (N.D., unpublished data). Taken together, the observed testicular growth, seminiferous tubule enlargement, reduction of spermatogonia at week 12 and increase of spermatids and luminal spermatozoa, indicate that the rFsh/rLh treatment was effective in stimulating spermiogenesis and spermiation.
Although the role of Fsh and Lh in the reproductive process of fish has not yet been fully elucidated, it is generally accepted that Fsh acts on the early events of fish gametogenesis, whereas Lh acts on the final events of this process49. Hence, the administration of rFsh and rLh according to the protocol reported earlier42, followed by the one used in the present study, appeared to mimic efficiently the physiological activation of the reproductive axis that occurs at the onset of sexual maturation. In fact, the first phase of the treatment, based only on rFsh administration, was able to stimulate the onset of gametogenesis, characterised by the transition from spermatogonial stem cell self-renewal to rapid proliferation towards meiosis42, whereas the second phase of the treatment, with both rFsh and rLh in the present study, was able to support the last events of spermatogenesis, i.e. spermatid differentiation into spermatozoa and spermiation.
The treatments with rFsh followed by the combination of rFsh/rLh were highly effective in the stimulation of all phase of spermatogenesis, including proliferation of committed spermatogonia, entry in meiosis, spermiogenesis and spermiation. However, the timing of the experimental design did not allow demonstrating any effect on spermatogonial stem cell self-renewal. In fact, the initial treatment with rFsh likely stimulated spermatogonial self-renewal, but this occurred for a limited period after the first rFsh administration(s) and was not detected by our earlier study42. The time-limited stimulation of spermatogonial stem cell self-renewal and the consequent reduction of stem germ cell reservoir might have limited the growth of testes in rFsh/rLh-treated juvenile meagre, which did not attain peak GSI values recorded in adults. However, high GSI values and/or sustained spermiation were reported in Japanese eel Anguilla japonica50, European sea bass Dicentrarchus labrax51, yellowtail kingfish Seriola lalandi52, Senegalese sole36 and flathead grey mullet41 that underwent different treatments with rGths, suggesting that spermatogonial stem cell self-renewal did not represent a limitation to testicular growth and spermatogenesis. These apparently contradictory data suggest the need to conduct further studies to fully elucidate the mechanisms regulating spermatogonial self-renewal and the role exerted by Fsh/Lh on this process.
The presence of apoptotic cells in adult testis has been reported widely in all vertebrate classes, from fish to mammals7,9,44,53,54,55,56,57,58,59,60,61,62. In vertebrates with seasonal reproductive cycles, an increase of apoptosis is observed commonly during testicular regression at the end of the reproductive season, concomitantly with the withdrawal of Fsh/Lh and sex steroids release, which act as survival factors for germ cells, while little testicular apoptosis is observed during recrudescence or the breeding season44,62,63,64,65,66. In teleost fishes, the limited available data suggest species-specific differences in the role of testicular apoptosis and the existence of mechanisms regulating the balance between germ cell proliferation and apoptosis. Germ cell apoptosis in fish can occur during different stages: in zebrafish Danio rerio67, guppies Poecilia reticulata68 and Atlantic cod Gadus morhua69, the main loss of germ cells occurs in the spermatogonial phase, whereas in tilapia Oreochromis niloticus it is mainly observed during spermiogenesis70. In Atlantic salmon Salmo salar, the loss of stimulated by retinoic acid gene 8 protein (stra8) gene expression induced an increase of germ cell apoptosis, compensated by an elevated production of spermatogenic cysts71. A high level of spermatogonial apoptosis was reported in untreated pre-pubertal meagre and treatment with rFsh resulted in a significant decrease of apoptotic cell death42. It was supposed that the high level of apoptosis observed in pre-pubertal meagre was responsible for the removal of germ cells than cannot proceed towards spermatogenesis, due to insufficient levels of Fsh/Lh and sex steroids and that the 6-week treatment with rFsh was able to eliminate the apoptotic block, allowing spermatogonia to proliferate and enter in meiosis42. In the present study, apoptosis involved Sertoli cells and spermatogonia, and increased markedly in treated fish sampled at week 21, suggesting that the cessation of the treatment at week 12 induced extensive apoptosis of germ cells -and associated Sertoli cells- that could not proceed with spermatogenesis/spermiogenesis due to the withdrawal of Fsh/Lh. The present apoptosis data agree with another study that reported that insulin-like 3 (Insl3), a Leydig cell-derived growth factor whose expression is up-regulated by Fsh, stimulates the differentiating proliferation of type A undifferentiated spermatogonia, and loss of Insl3 triggers germ cell apoptosis in male zebrafish72. Moreover, the present study is in agreement with previous studies showing that reproductively dysfunctional adult Atlantic bluefin tuna Thunnus thynnus under captivity-induced chronic stress had high level of testicular apoptosis6,7,55, and Fsh/Lh release from the pituitary after the administration of gonadotropin releasing hormone agonist provoked a significant decrease of apoptosis cells55.
Conclusions
A two-step protocol based on the administration of rFsh42 followed by a combination of rFsh and rLh (present study) proved to be effective in inducing the completion of puberty precociously in meagre, and resulting in the production of releasable sperm. The first step42, effectively removed the apoptotic block that prevents spermatogonia to proceed towards spermatogenesis, and stimulated testis growth, proliferation of committed spermatogonia and entering in meiosis. The second step (present study) resulted in the persisting survival of germ cells, further stimulated testis growth and induced spermiogenesis and spermiation. The proposed protocol proved to be effective in advancing reproductive maturation in male meagre, and laid the bases to reduce the generation time of selective breeding programs. Further studies are needed to verify the superiority of recombinant gonadotropin technology over conventional hormone treatments for the induction of spermatogenesis in pre-pubertal meagre.
Data availability
Fsh α and β subunits amino acid sequences were deduced from the meagre pituitary mRNA sequences deposited in the European Nucleotide Archive (ENA) under the project accession number PRJEB57583. All the other data produced and/or analyzed during the current study are included in this article and in Supplementary Information file.
References
Chao, L. N. & Trewavas, E. Sciaenidae in Check-list of the fishes the eastern tropical Atlantic (CLOFETA) Vol. 2 (eds. Quero, J. C., Hureau, J. C., Karrer, C., Post, A. & Saldanha, L.) 813–826 (JNICT, Lisbon; SEI, Paris; and UNESCO, Paris, 1990).
EUMOFA, European Market Observatory for Fisheries and Aquaculture Products-Meagre in the EU. ISBN 978-92-76-47640-5 (2022). https://www.eumofa.eu Accessed 12 June 2023.
FAO, Global aquaculture production Quantity. License: CC BY-NC-SA 3.0 IGO. Extracted from (1950–2020). https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_quantity (2022). (Accessed 12 June 2023)
Mylonas, C. C. et al. Multiple spawning and egg quality of individual European sea bass (Dicentrarchus labrax) females after repeated injections of GnRHa. Aquaculture 221, 605–620. https://doi.org/10.1016/S0044-8486(03)00120-0 (2003).
Mylonas, C. C. et al. Induction of spawning of cultured greater Amberjack (Seriola dumerili) using GnRHa implants. Aquaculture 237, 141–154. https://doi.org/10.1016/j.aquaculture.2004.04.015 (2004).
Zupa, R. et al. Comparative analysis of male germ cell proliferation and apoptosis in wild and captive Atlantic Bluefin tuna (Thunnus Thynnus L). J. Appl. Ichthyol. 29, 71–81. https://doi.org/10.1111/j.1439-0426.2012.02045.x (2013).
Zupa, R. et al. Male germ cell proliferation and apoptosis during the reproductive cycle of captive-reared Atlantic Bluefin tuna Thunnus thynnus (Linnaeus). Aquac Res. 45, 1733–1736. https://doi.org/10.1111/are.12110 (2014).
Zupa, R. et al. Comparative study of reproductive development in wild and captive-reared greater Amberjack Seriola dumerili (Risso, 1810). PLoS ONE. 12 (1), e0169645. https://doi.org/10.1371/journal.pone.0169645 (2017).
Zupa, R. et al. Rearing in captivity affects spermatogenesis and sperm quality in greater Amberjack Seriola dumerili (Risso, 1810). J. Anim. Sci. 95, 4085–4100. https://doi.org/10.2527/jas.2017.1708 (2017).
Pousis, C. et al. The observed oogenesis impairment in greater Amberjack Seriola dumerili (Risso, 1810) reared in captivity is not related to an insufficient liver transcription or oocyte uptake of vitellogenin. Aquac Res. 49, 243–252. https://doi.org/10.1111/are.13453 (2018).
Pousis, C. et al. Vitellogenin receptor and fatty acid profiles of individual lipid classes of oocytes from wild and captive-reared greater Amberjack (Seriola dumerili) during the reproductive cycle. Theriogenology 140, 73–83. https://doi.org/10.1016/j.theriogenology.2019.08.014 (2019).
Fakriadis, I. & Mylonas, C. C. Sperm quality of greater Amberjack Seriola dumerili throughout the reproductive season and in response to GnRHa treatment with controlled release implants. Fish. Physiol. Biochem. 47, 281–292. https://doi.org/10.1007/s10695-020-00910-9 (2021).
Lavecchia, A. et al. Dysregulation of testis mRNA expression levels in hatchery-produced vs wild greater Amberjack Seriola dumerili. Sci. Rep. 13, 13662. https://doi.org/10.1038/s41598-023-40597-5 (2023).
Lavecchia, A. et al. Comparison of ovarian mRNA expression levels in wild and hatchery–produced greater Amberjack Seriola dumerili. Sci. Rep. 14, 18034. https://doi.org/10.1038/s41598-024-69091-2 (2024).
Duncan, N. et al. Reproductive development, GnRHa-induced spawning and egg quality of wild meagre (Argyrosomus regius) acclimatised to captivity. Fish. Physiol. Biochem. 38, 1273–1286. https://doi.org/10.1007/s10695-012-9615-3 (2012).
Duncan, N. J. et al. Aquaculture, production of meagre (Argyrosomus regius), hatchery techniques, ongrowing and market. in Advances in Aquaculture Hatchery Technology (eds Allan, G. & Burnell, G.) 519–541 (Woodhead Publishing Limited, Cambridge, UK, 2013).
Duncan, N. J. et al. Paired spawning with male rotation of meagre Argyrosomus regius using GnRHa injections, as a method for producing multiple families for breeding selection programs. Aquaculture 495, 506–512. https://doi.org/10.1016/j.aquaculture.2018.06.017 (2018).
Fernandez-Palacios, H. et al. Dose-dependent effect of a single GnRHa injection on the spawning of meagre (Argyrosomus regius) broodstock reared in captivity. Span. J. Agri Res. 12, 1038–1048. https://doi.org/10.5424/sjar/2014124-6276 (2014).
Mylonas, C. C. et al. Reproduction of hatchery-produced meagre Argyrosomus regius in captivity II. Hormonal induction of spawning and monitoring of spawning kinetics, egg production and egg quality. Aquaculture 414–415, 318–327. https://doi.org/10.1016/j.aquaculture.2013.09.008 (2013).
Mylonas, C. C., Mitrizakis, N., Papadaki, M. & Sigelaki, I. Reproduction of hatchery-produced meagre Argyrosomus regius in captivity I. Description of the annual reproductive cycle. Aquaculture 414–415, 309–317. https://doi.org/10.1016/j.aquaculture.2013.09.009 (2013).
Mylonas, C. C. et al. Reproduction of hatchery-produced meagre Argyrosomus regius in captivity III. Comparison between GnRHa implants and injections on spawning kinetics and egg/larval performance parameters. Aquaculture 448, 44–53. https://doi.org/10.1016/j.aquaculture.2015.05.036 (2015).
Mylonas, C. C. et al. Enhancement of oogenesis/spermatogenesis in meagre Argyrosomus regius using a combination of temperature control and GnRHa treatments. Aquaculture 464, 323–330. https://doi.org/10.1016/j.aquaculture.2016.07.006 (2016).
Ramos-Júdez, S. et al. Gamete quality and management for in vitro fertilisation in meagre (Argyrosomus regius). Aquaculture 509, 227–235. https://doi.org/10.1016/j.aquaculture.2019.05.033 (2019).
Zupa, R. et al. Reproductive maturation of meagre Argyrosomus regius (Asso, 1801) reared in floating cages. Animals 13, 223. https://doi.org/10.3390/ani13020223 (2023).
Carrillo, M. et al. Hormonal and environmental control of puberty in perciform fish: The case of sea bass. Ann. N Y Acad. Sci. 1163, 49–59. https://doi.org/10.1111/j.1749-6632.2008.03645.x (2009).
Taranger, G. L. et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 165, 483–515. https://doi.org/10.1016/j.ygcen.2009.05.004 (2010).
Schulz, R. W. et al. Spermatogenesis in fish. Gen. Comp. Endocrinol. 165, 390–411. https://doi.org/10.1016/j.ygcen.2009.02.013 (2010).
Holland, M. C. H., Hassin, S. & Zohar, Y. Effects of long-term testosterone, gonadotropin-releasing hormone agonist, and Pimozide treatments on gonadotropin II levels and ovarian development in juvenile female striped bass (Morone saxatilis). Biol. Reprod. 59, 1153–1162. https://doi.org/10.1095/biolreprod59.5.1153 (1998).
Klenke, U. & Zohar, Y. Gonadal regulation of gonadotropin subunit expression and pituitary LH protein content in female hybrid striped bass. Fish. Physiol. Biochem. 28, 25–27. https://doi.org/10.1023/B:FISH.0000030465.56876.97 (2004).
Klenke, U. & Zohar, Y. Direct pituitary regulation of brain and pituitary reproductive hormone expression by estrogen: An in vitro study. 5th Intl. Symp. Fish Endocrinol. Sept. 5–9, (2004).
Klenke, U. & Zohar, Y. Seasonal hormonal regulation of the reproductive brain: An in vitro brain-slice study. 15th Intl. Congr. Comp. Endocrinol. May 22–27, (2005).
Chu, L., Li, J., Liu, Y. & Cheng, C. H. Gonadotropin signaling in zebrafish ovary and testis development: Insights from gene knockout study. Mol. Endocrinol. 29, 1743. https://doi.org/10.1210/ME.2015-1126 (2015).
Nobrega, R. et al. (ed, H.) Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology 156 3017–3084 https://doi.org/10.1210/en.2015-1157 (2015).
González-Cid, Á., Giménez, I. & Duncan, N. In vivo effect of Recombinant Fsh and Lh administered to meagre (Argyrosomus regius) at the initial stages of sex differentiation. Gen. Comp. Endocrinol. 356, 114576. https://doi.org/10.1016/j.ygcen.2024.114576 (2024).
Lancerotto, S. et al. Overcoming dysfunctional gametogenesis in hatchery-produced greater Amberjack, Seriola dumerili using recombinant gonadotropins, and spawning induction using gonadotropin releasing hormone agonist-loaded implants. Aquaculture 594, 741401. https://doi.org/10.1016/j.aquaculture.2024.741401 (2024).
Chauvigné, F. et al. Toward developing Recombinant gonadotropin-based hormone therapies for increasing fertility in the flatfish Senegalese sole. PLoS ONE 12, e0174387. https://doi.org/10.1371/journal.pone.0174387 (2017).
Chauvigné, F. et al. Seasonal-and dose-dependent effects of recombinant gonadotropins on sperm production and quality in the flatfish Solea senegalensis. Comp. Biochem. Physiol. Mol. Integr. Physiol. 225, 59–64. https://doi.org/10.1016/j.cbpa.2018.06.022 (2018).
Peñaranda, D. et al. Using specific recombinant gonadotropins to induce spermatogenesis and spermiation in the European eel (Anguilla anguilla). Theriogenology 107, 6–20. https://doi.org/10.1016/j.theriogenology.2017.11.002 (2018).
Jéhannet, P. et al. Recombinant gonadotropins to induce oocyte development in vitro and in vivo in the European eel Anguilla anguilla. Fishes 8, 123. https://doi.org/10.3390/fishes8030123 (2023).
Ramos-Júdez, S. et al. Providing recombinant gonadotropin-based therapies that induce oogenesis from previtellogenic oocytes to produce viable larvae in a teleost, the flathead grey mullet (Mugil cephalus). Aquaculture 536, 736418. https://doi.org/10.1016/j.aquaculture.2021.736418 (2021).
Ramos–Júdez, S. et al. Recombinant Fsh and Lh therapy for spawning induction of previtellogenic and early spermatogenic arrested teleost, the Flathead grey mullet (Mugil cephalus). Sci. Rep. 12, 6563. https://doi.org/10.1038/s41598-022-10371-0 (2022).
Zupa, R. et al. Male germ cell proliferation and apoptosis in sexually immature meagre Argyrosomus regius (Asso, 1801) treated with Recombinant follicle stimulating hormone. Sci. Rep. 13, 7013. https://doi.org/10.1038/s41598-023-34102-1 (2023).
Zupa, R., Martino, N. A., Marzano, G., Dell’Aquila, M. E. & Corriero, A. Meagre Argyrosomus regius (Asso, 1801) stem spermatogonia: histological characterization, immunostaining, in vitro proliferation, and cryopreservation. Animals 10, 851. https://doi.org/10.3390/ani10050851 (2020).
Young, K. A. & Nelson, R. J. Mediation of seasonal testicular regression by apoptosis. Reproduction 122, 677–685. https://doi.org/10.1530/rep.0.1220677 (2001).
Sokal, R. R. & Rohlf, F. J. Biometry The Principles and Practice of Statistics in Biological Research (Freeman WH and Company, 1981).
Mylonas, C. C. & Zohar, Y. Use of GnRHa-delivery systems for the control of reproduction in fish. Rev. Fish. Biol. Fisher. 10, 463–491. https://doi.org/10.1023/A:1012279814708 (2001).
Mylonas, C. C., Fostier, A. & Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 165, 516–534. https://doi.org/10.1016/j.ygcen.2009.03.007 (2010).
Kumakura, N., Okuzawa, K., Gen, K. & Kagawa, H. Effects of gonadotropin-releasing hormone agonist and dopamine antagonist on hypothalamus-pituitary-gonadal axis of pre-pubertal female red seabream (Pagrus major). Gen. Comp. Endocrinol. 131, 264–273. https://doi.org/10.1016/S0016-6480(03)00012-1 (2003).
Allan, C. M. et al. Complete Sertoli cell proliferation induced by follicle stimulating hormone (FSH) independently of luteinizing hormone activity: Evidence from genetic models of isolated FSH action. Endocrinology 145, 1587–1593. https://doi.org/10.1210/en.2003-1164 (2004).
Kamei, H., Kaneko, T. & Aida, K. Vivo gonadotropic effects of recombinant Japanese eel follicle-stimulating hormone. Aquaculture 261, 771e5. https://doi.org/10.1016/j.aquaculture.2006.08.039 (2006).
Mazón, M. J., Gómez, A., Yilmaz, O., Carrillo, M. & Zanuy, S. Administration of follicle-stimulating hormone in vivo triggers testicular recrudescence of juvenile European sea bass (Dicentrarchus labrax). Biol. Reprod. 90, 6. https://doi.org/10.1095/biolreprod.113.110569 (2014).
Sanchís-Benlloch, P. J. et al. In-vitro and in-vivo biological activity of Recombinant Yellowtail kingfish (Seriola lalandi) follicle stimulating hormone. Gen. Comp. Endocrinol. 241, 41–49. https://doi.org/10.1016/j.ygcen.2016.03.001 (2017).
Prisco, M. et al. Apoptosis during spermatogenesis in the spotted ray Torpedo marmorata. Mol. Reprod. Dev. 64, 341–348. https://doi.org/10.1002/mrd.10267 (2003).
Corriero, A. et al. Germ cell proliferation and apoptosis during different phases of swordfish (Xiphias gladius L.) spermatogenetic cycle. J. Fish. Biol. 70, 83–99. https://doi.org/10.1111/j.1095-8649.2006.01257.x (2007).
Corriero, A. et al. Proliferation and apoptosis of male germ cells in captive Atlantic Bluefin tuna (Thunnus Thynnus L.) treated with gonadotropin-releasing hormone agonist (GnRHa). Anim. Reprod. Sci. 116, 346–357. https://doi.org/10.1016/j.anireprosci.2009.02.013 (2009).
Ventriglia, G. et al. Effects of different hormonal treatments on spermatogenesis advancement in hatchery-produced greater Amberjack Seriola dumerili (Risso 1810). Gen. Comp. Endocrinol. 348, 114447. https://doi.org/10.1016/j.ygcen.2024.114447 (2024).
Jenkins, L. K., Ross, W. L. & Young, K. A. Increases in apoptosis and declines in Bcl-XL protein characterise testicular regression in American crows (Corvus brachyrhynchos). Reprod. Fertil. Dev. 19, 461–469. https://doi.org/10.1071/rd06079 (2007).
Wang, D. H. et al. The apoptotic function analysis of p53, Apaf1, Caspase3 and Caspase7 during the spermatogenesis of the Chinese fire-bellied Newt Cynops orientalis. PLoS ONE. 7, e39920. https://doi.org/10.1371/journal.pone.00399207 (2012).
Scaia, M. F., Czuchlej, S. C., Cervino, N. & Ceballos, N. R. Apoptosis, proliferation and presence of estradiol receptors in the testes and bidder’s organ of the Toad Rhinella arenarum (Amphibia, Anura). J. Morphol. 277, 412–423. https://doi.org/10.1002/jmor.20507 (2016).
Liu, T. et al. Molecular and cellular mechanisms of apoptosis during dissociated spermatogenesis. Front. Physiol. 8, 188. https://doi.org/10.3389/fphys.2017.00188 (2017).
Chen, H. et al. Characteristics of seasonal spermatogenesis in the soft-shelled turtle. Anim. Reprod. Sci. 214, 106307. https://doi.org/10.1016/j.anireprosci.2020.106307 (2020).
Valentini, L., Zupa, R., Pousis, C., Cuko, R. & Corriero, A. Proliferation and apoptosis of cat (Felis Catus) male germ cells during breeding and non-breeding seasons. Vet. Sci. 9, 447. https://doi.org/10.3390/vetsci9080447 (2022).
Blottner, S., Hingst, O. & Meyer, H. H. D. Inverse relationship between testicular proliferation and apoptosis in mammalian seasonal breeders. Theriogenology 44, 320–328. https://doi.org/10.1016/0093-691X(95)00187-D (1995).
Nandi, S., Banerjee, P. P. & Zirkin, B. R. Germ cell apoptosis in the testes of Sprague Dawley rats following testosterone withdrawal by ethane 1,2-dimethanesulfonate administration: Relationship to fas? Biol. Reprod. 61, 70–75. https://doi.org/10.1095/biolreprod61.1.70 (1999).
Woolveridge, I. et al. Apoptosis in the rat spermatogenic epithelium following androgen withdrawal: Changes in apoptosis-related genes. Biol. Reprod. 60, 461–470. https://doi.org/10.1095/biolreprod60.2.461 (1999).
Štrbenc, M., Fazarinc, G., Bavdek, V. & Pogačnik, A. Apoptosis and proliferation during seasonal testis regression in the brown hare (Lupus Europaeus L). Anat. Histol. Embryol. 32, 48–53. https://doi.org/10.1046/j.1439-0264.2003.00437.x (2003).
Leal, M. C. et al. Histological and Stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod. 81, 177–187. https://doi.org/10.1095/biolreprod.109.076299 (2009).
Billard, R. La spermatogenèse de Poecilia reticulata. I–estimation du Nombre de générations goniales et rendement de La spermatogenèse. Ann. Biol. Anim. Biochim. Biophys. 9, 251–271. https://doi.org/10.1051/rnd:19690208 (1969).
Almeida, F. F. L., Kristoffersen, C., Taranger, G. L. & Schulz, R. W. Spermatogenesis in Atlantic Cod (Gadus morhua): A novel model of cystic germ cell development. Biol. Reprod. 78, 27–34. https://doi.org/10.1095/biolreprod.107.063669 (2008).
Vilela, D. A. R., Silva, S. G. B., Peixoto, M. T. D., Godinho, H. P. & França, L. R. Spermatogenesis in teleost: Insights from the nile tilapia (Oreochromis niloticus) model. Fish. Physiol. Biochem. 28, 187–190. https://doi.org/10.1023/B:FISH.0000030523.16010.62 (2003).
Skaftnesmo, K. O. et al. Loss of stra8 increases germ cell apoptosis but is still compatible with sperm production in Atlantic salmon (Salmo salar). Front. Cell. Dev. Biol. 9, 657192. https://doi.org/10.3389/fcell.2021.657192 (2021).
Crespo, D. et al. Insulin-like 3 affects zebrafish spermatogenic cells directly and via sertoli cells. Commun. Biol. 4, 204. https://doi.org/10.1038/s42003-021-01708-y (2021).
Acknowledgements
For fish maintenance and sampling we thank Esteban Hernandez, Pol Moreno, Magda Monllaó, Olga Bellot, Marta Sastre and all IRTA technicians involved and also Zohar Ibarra and Joel Linares visiting from the Autonomous University of Nayarit, Mexico. Thank you to Ana Mendes, Pedro Pousão and IPMA, Olhão, Portugal for supplying the fish. Thank you to Marta Gut and Jèssica Gómez-Garrido from the Centre for Genomic Regulation (CRG), Barcelona, for sequencing of the Gth genes. Thank you also to Dr. Deborah Maria Del Frassino and Alessandro Papaleo for their technical support and text formatting.
Funding
The present work was funded by the project NewTechAqua (European Union´s Programme H2020, GA 862658) awarded to N.D., C.C.M., I.G. and A.C.
Author information
Authors and Affiliations
Contributions
G.V.: generated semi-quantitative data, prepared the figures and wrote the first draft of the manuscript. N.D.: conceived and designed the experiment, wrote the first draft of the manuscript. I.G.: produced recombinant gonadotropins, designed the experiment and wrote the first draft of the manuscript. C.C.M.: designed the experiment and critically revised the first draft of the manuscript. C.P.: collected and prepared samples for laboratory analysis, performed TUNEL analysis. A.C.: designed the experiment, interpreted data, and wrote the first draft of the manuscript. R.Z.: performed statistical analyses, interpreted data, and wrote the first draft of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Gianluca Ventriglia, Neil Duncan, Constantinos C. Mylonas, Chrysovalentinos Pousis, Aldo Corriero and Rosa Zupa declare no competing interest. Ignacio Gimenéz is associated with the biotech company Rara Avis Biotech, S. L., which produced the recombinant gonadotropin employed in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ventriglia, G., Duncan, N., Giménez, I. et al. Spermatogenesis advancement in pre-pubertal meagre Argyrosomus regius treated with recombinant gonadotropins. Sci Rep 15, 15113 (2025). https://doi.org/10.1038/s41598-025-99372-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99372-3