Abstract
In this study, we present new ancient DNA data from prehistoric and historic populations of the Iranian Plateau. By analysing 50 samples from nine archaeological sites across Iran, we report 23 newly sequenced mitogenomes and 13 nuclear genomes, spanning 4700 BCE to 1300 CE. We integrate an extensive reference sample set of previously published ancient DNA datasets from Western and South-Central Asia, enhancing our understanding of genetic continuity and diversity within ancient Iranian populations. A new Early Chalcolithic sample, predating all other Chalcolithic genomes from Iran, demonstrates mostly Early Neolithic Iranian genetic ancestry. This finding reflects long-term cultural and biological continuity in and around the Zagros area, alongside evidence of some western genetic influence. Our sample selection prioritizes northern Iran, with a particular focus on the Achaemenid, Parthian, and Sassanid periods (355 BCE–460 CE). The genetic profiles of historical samples from this region position them as intermediates on an east-west genetic cline across the Persian Plateau. They also exhibit strong connections to local and South-Central Asian Bronze Age populations, underscoring enduring genetic connections across these regions. Diachronic analyses of uniparental lineages on the Iranian Plateau further highlight population stability from prehistoric to modern times.
Similar content being viewed by others
Introduction
The Persian Plateau in West Asia—defined here as a vast geographical area stretching from eastern Anatolia to Afghanistan and western parts of Pakistan, also including the south Caucasus, South-Central Asia, and north Mesopotamia1,2,3—played a significant role in shaping human prehistory, as a major hub for early Homo sapiens migration out of Africa1,4. Human populations colonized Eurasia, Oceania, and the Americas in multiple waves from this hub between 70/60 and 46 thousand years ago1. It is also recognized as a key region where the early diverging Basal Eurasian genetic lineage likely existed, described as an Upper Palaeolithic Homo sapiens genetic ancestry without admixture from Homo neanderthalensis, in contrast to other ancient non-Africans5. Prehistoric farmers, and later, ancient civilizations thrived on the Plateau, benefiting from its favorable geographic location and abundant resources, which fostered trade and intercultural relations6. Recent archaeogenetic studies have started to shed light on the complex nature of these ancient populations who inhabited the Persian Plateau5,7,8,9,10,11,12,13,14,15,16,17. Mesolithic hunter-gatherer (HG) remains from the Alborz Mountains in northern Iran show ancestries primarily related to the Basal Eurasians5,12. The Early Neolithic (EN) farmers from Ganj Dareh are proposed to be descendants of these Iranian HGs16 and are genetically cladal with the Caucasus HGs (CHG)15. These groups that descend from the inferred Upper Palaeolithic period populations of Western Asia are a focal point for subsequent comparative genetic studies4,7,8,15,16. They carry distinct genetic components compared to the Neolithic farmers of the Levant and Anatolia, as well as the European HGs5. Further investigations revealed that the Chalcolithic farmers in both the western and eastern Iranian Plateau5,16 (defined here as the current territory of Iran) have additional ancestries related to the Natufian and Pre-Pottery Neolithic (PPN) Levant besides the Anatolian Neolithic Farmers (ANF)13. As the Chalcolithic period concluded and the Bronze Age (BA) began, more varied genetic components entered the ancient Iranian gene pool from the east and north, including the Andamanese hunter-gatherer-related (AHG), Ancient North Eurasian-related (ANE), and BA Steppe-related ancestries5,12,14,16. AHG-related ancestry is first detected in the region as an influx from South Asia (Indus Valley) at the BA Shahr-i Sokhta in eastern Iran12, a major population center around 3550–2900 BCE18 and a civilization center up to 2300 BCE (Supplementary Information). ANE-related ancestry is recognized in West Siberian Hunter-Gatherers (WSHG) and the Central-Asian Eneolithic/Chalcolithic Botai culture’s people, with genetic continuity from more archaic ANE populations of Northern Asia19. BA Steppe-related ancestry, which shares genetic ancestry with the CHG, and also with the ANE-related groups through its East European HG components, is often associated with the Yamnaya culture’s people and descendant groups who spread across the Eurasian continent from the Pontic Steppe12. The diversity and integration of various genetic components in the Iranian Plateau became even more pronounced during the Iron Age (IA), evident in continuous Levant-related gene flow at this period13.
Despite this progress, our understanding of past genetic events remains incomplete as many populations are still uncharacterized, and the extensive human mobility in historical periods further complicates the genetic landscape20. This highlights the importance of studying the prehistoric and historical genetic makeup of the region to better understand cultural and population developments in later periods. This is particularly relevant for the northeastern and western regions of the Iranian Plateau, two areas with extensive connections to South Central Asia (including Turkmenistan, Uzbekistan, and Tajikistan) and South Caucasus (Azerbaijan, Armenia, and Georgia), respectively, from the Neolithic period to the historical times6,21 (see Supplementary information for more details).
These connections further expanded under the first Persian Empire, the Achaemenids, whose core territories included the Iranian Plateau, South Caucasus, South Central Asia, and regions of modern Afghanistan and Pakistan. In 323 BCE, the Seleucid Empire emerged as a Hellenistic state encompassing most of the Iranian Plateau22. By the mid-3rd century BCE, the warriors of the Aparni or Parni—a nomadic or semi-nomadic eastern Iranian tribe—rose to power and established the Parthian government in Nisa, from where they extended their control over the Persian Plateau. Accounts of the Parthian Empire and its tribes are documented in the works of Herodotus and Strabo23,24.
The catacomb type graves, also typical for the Parthians, were common throughout the Persian Plateau until the end of the BA. The oldest catacomb graves, dated to 3000–2900 BCE and excavated in the Jiroft area, predate those at Shahr-i Sokhta (after 2700 BCE)25 and in South-Central Asia (e.g., Turkmenistan). This suggests that early cultures of southeastern Iran influenced the burial practices in Baluchistan and southern Turkmenistan26. This tradition re-emerged during the Parthian and Sassanid periods in the north and northeastern Iranian Plateau (i.e., Liarsangbon and Vestemin, see Supplementary Information, Chaps. 8 and 9, for more details).
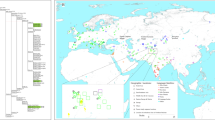
The objective of our research is to conduct a genetic analysis of key archaeological sites in Iran, from the Chalcolithic period to the Medieval era. We focus particularly on the Achaemenid, Seleucid, Parthian, and Middle Sassanid periods (355 BCE-460 CE) in northern Iran, given its strategic location as a migration and trade corridor for millennia. We investigate three historical-period sites, and provide the first genetic evaluation of the northern Iranian Plateau “highway”, a key section of the Silk Roads27 that facilitated the distribution of diverse materials and artefacts south of the Caspian Sea (Fig. 1).
Using genomic data, we aim to uncover genetic variation, as well as possible continuity and change among ancient Iranian populations and their connections to neighbouring populations (e.g., in the South-Caucasus and South-Central Asia), by comparing genetic and archaeological records across different regions. Our analyses seek to explore whether and how human movement, historical events, and cross-cultural interactions, such as trade, have influenced the genetic makeup of the Plateau’s populations over time.
Map of the Iranian Plateau and its surroundings. Present-day Iran is highlighted, with the archaeological sites mentioned in the text. Filled circles represent new samples, empty circles mark sites from which ancient DNA data has been published in previous studies5,7,8,9,10,11,12,13,14,15,17. For further sites that were processed but not analysed, refer to Supplementary Table S1.
Results and discussion
Based on initial screening and shallow shotgun sequencing of 50 human DNA samples, five samples met the criteria for direct deeper shotgun sequencing (Methods), which resulted in genomes with ~ 0.20–1.11× average genomic coverage (mean ~ 0.61×). The number of detected SNPs from the 1240k panel28 varies between 189k and 741k (mean ~ 462k).
Initially, we used MyBaits Arbor Complete panel for genomic and mitogenome capture for other samples. However, following a critical review of this method29, we repeated the target enrichment with Twist capture in seven cases30 to validate the results. Overall, eight samples were processed with MyBaits Arbor capture, with an average of ~ 164k SNPs detected from the 1240k SNP set. For the Twist-captured samples, an average of ~ 182k SNPs was covered, ranging between 11k and 770k SNPs. Finally, we sequenced 14 mitochondrial genomes after a separate mitogenome capture for samples from the Gohar Tepe, Marsin Chal, Liarsangbon, Vestemin, and Kalmakareh archaeological sites (see Methods, Table 1, Supplementary Tables S1-S4). The mean mtDNA coverage was 44.57×.
We used the BREADR package (Biological Relatedness from Ancient DNA in R) to assess kinship among the whole-genome data samples and found no close relatedness with sufficient SNP overlap between the compared individuals32. The only exception is a pair of genetically identical/twin individuals (IRN23 and IRN25) from Liarsangbon (Supplementary Table S8). Anthropological records confirm that these two individuals are twins (Supplementary Information, Chap. 8).
Analysing possible parental relatedness in the new genome-wide dataset from Iran, we detected varying runs of homozygosity (ROH)33 signals among individuals from different archaeological sites (Supplementary Table S15, Figure S1). Individual IRN22 from Liarsangbon stands out with the highest ROH, characterized by long homozygous runs (> 20 cM) indicating a high degree of inbreeding, likely due to parents who were first- or second-degree relatives33. In contrast, individuals IRN24 from Gol Afshan Tepe, IRN57 from Mersin Chal, IRN23 from Liarsangbon and IRN02 from Vestemin show minimal ROH, whereas others have nearly no signal, suggesting a more outbred ancestry and a relatively large mating pool (Supplementary Figure S1). Comparing the current dataset with previous analyses of this kind, we infer that signals of consanguinity are sporadically present across the broader Early Neolithic Iranian Plateau (Ganj Dareh, Tepe Abdul Hosein, Wezmeh) and in the Turkmenistan areas, which had cultural contact with Iranian Plateau, including Chalcolithic sites such as Namazga and Parkhai21,34 (Supplementary Table S15). Evidence of small effective population sizes are also apparent at the Chalcolithic to Iron Age sites Tepe Hissar, Shah Tepe, Dinkha Tepe, and Hasanlu of the Iranian Plateau, as well as BA Turkmenistan. Considering the available Iranian and Turkmenistan prehistoric datasets, we conclude that the effective population size increased over time from the Neolithic to the Iron Age, where the Neolithic mating pool was 3–6 times smaller than in later periods (Supplementary Table S15).
A diachronic overview and frequency-based comparisons of the maternal lineages in the Iranian Plateau
To our knowledge, 127 ancient mitogenomes have been previously published from 20 archaeological sites on the Iranian Plateau, spanning the Mesolithic to Medieval times (Supplementary Table S5). We merged our new mtDNA results obtained from three different laboratory approaches (n = 23), with these mtDNA data. The combined dataset allowed us to identify 23 distinct maternal macro-haplogroups in the region (Fig. 2A).
A significant proportion of these ancient haplogroups correspond to the more ubiquitous West Eurasian lineages (70% in total), such as U, R, J, H, HV, and T, which are common even in the modern Iranian maternal gene pool35. In general, we detect a predominance of these maternal lineages on the Iranian Plateau during both prehistorical and historical periods. While the Neolithic-Chalcolithic periods show continuity to the BA on the maternal level, the latter period exhibits increasing variability with the appearance of several new mtDNA haplogroups. This trend is evident in the Shahr-i Sokhta dataset, which includes haplogroups such as L and M, R5 or T112, as well as the arrival of additional Eastern Eurasian lineages (A, B, C, D, F, R, U6, Y and Z) in the post-BA times. Our findings align with earlier archaeological descriptions of increased population mobility6,9. The Northwestern Iranian IA sample set9,12,13,17 is dominated by Western Eurasian haplogroups, however the IA also marks the introduction of Eastern Asian haplogroup D to the Iranian Plateau (Sagzabad site in the northern Qazvin province)11,17. These patterns are consistent with the mtDNA gene pool of southern Turkmenistan during the Chalcolithic - Bronze Age (except haplogroup L), which was connected geographically and culturally to the eastern Iranian Plateau. The historical sample set shows the abundance of J1, along with an absence of R and Eastern Eurasian mitochondrial haplogroups.
We conducted principal component analysis (PCA) on the maternal haplogroup frequencies derived from a dataset of the first hypervariable region of the mtDNA (Supplementary Table S5-6, Fig. 2B). Our data comprises samples from 19 modern Iranian groups (n = 1,498)35, and some representative ancient populations from different periods of Iran, such as EN Ganj Dareh, Chalcolithic Tepe Hissar, BA Shahr-i Sokhta, IA Hasanlu, and the newly-analysed historical samples from the Marsin Chal, Liarsangbon, and Vestemin sites in northern Iran.
Summary of the uniparental data. (A) Distribution of the ancient Iranian mtDNA haplogroups; asterisks denote the samples analysed in this study. (B) PCA plot based on mtDNA haplogroup frequencies in modern Iranian and selected ancient Iranian groups. Using the frequencies of 36 haplogroups in modern and ancient Iranian populations, the first two principal components (PCs) capture 22.1% of the total variance (see Supplementary Table S6 for details). To maintain consistency and prevent sampling bias, we selected only those ancient groups that had more than 10 individuals or could be merged geographically and temporally, such as our historical samples. (C) Distribution of the ancient Iranian Y-chromosomal haplogroups (details in Supplementary Table S7).
Our mtDNA analyses reveal a distinct demarcation along PC1 between BA Shahr-i Sokhta samples, and other Iranian and Armenian ancient populations (Fig. 2B). The new historical samples cluster together with EN Ganj Dareh, whereas Chalcolithic Tepe Hissar and IA Hasanlu are also positioned closely on the mtDNA PCA. Notably, some modern Iranian groups align with our historical samples along PC2, particularly the Iranian Armenians and Lurs, who share similar frequencies of haplogroups H and HV, and elevated proportions of K and J1 (Supplementary Figure S10.1). The new Medieval sample set from Kalmakareh demonstrates the local continuity of N and HV lineages in Lurestan Province through to the modern era35, where Iranian Bakhtiari nomadic people (with 51% N and 15% HV frequencies) have been recorded to inhabit for the last 800 years36. Trends in the mitochondrial gene pool are described in detail in Supplementary Information, Chap. 10 (Supplementary Table S5-6).
Y-chromosomal haplogroup distribution
To achieve an overview of the paternal genomic composition of the region, we combined the new Y-chromosomal results (n = 7) with data from previously published Mesolithic to historical-period males (n = 61), originating from 13 different archaeological sites in Iran (Supplementary Table S7 and Fig. 2C). The Y-chromosomal variation among the ancient Iranian populations mainly consisted of haplogroups J, G, L, R and T. The presence of the South Asian macro-haplogroup H, detected in Late Chalcolithic (Tepe Hissar) and Bronze Age (Shahr-i Sokhta) sites, aligns with the previously described cross-regional interactions characteristic of the Indo-Iranian borderland area12,13 (see more detail in Supplementary Information, Chap. 10). Haplogroups G, J, P1 and R2 were present on the Persian Plateau and its surroundings as early as the Mesolithic and Neolithic periods5,7,12,13. Our findings align with the previous research, indicating that these lineages have been present in the region for millennia. Haplogroup J1 is widely recognized to have originated approximately 20,000 years ago in a region encompassing western parts of the Persian Plateau13,37. In Iran, J1 can be traced back to the Bronze Age at the Hasanlu and Dinkha Tepe sites in the northwest13 (Supplementary Table S7). The evidence of its presence in the Plateau now extends into historical times with the newly analysed samples. Haplogroup J2 is considered to have formed approximately 31,600 years ago in a region covering northwestern and western Persian Plateau38. The frequency of J2 seems to have increased from the Neolithic era and it reached its peak during the Bronze Age, and remained at a relative high frequency into the historical periods in the Iranian Plateau, potentially due to greater interactions with regions to the west of the Plateu7,12,13 (Supplementary Table S7, Fig. 2). More details on the newly analysed dataset can be found in Supplementary Information, Chap. 10.
Population genomic trends from the Neolithic to historic periods in the Iranian Plateau
In order to compare the new Iranian sample set to the published ancient and modern Eurasian populations, we applied genomic PCA on ancient and modern Eurasian and North African samples from the AADR Human Origins (HO) dataset v5439, supplemented with other relevant published datasets (Supplementary Table S9). f4-statistics and qpAdm40,41 were performed on subsets of the AADR 1240k and HO dataset and other published ancient individuals (see Methods). We performed supervised ADMIXTURE42 on a subset of this merged dataset (n = 3913), as described in Methods, with results presented in Fig. 3, Supplementary Figure S11.3 and Supplementary Table S10.
Based on the observations by Davidson et al.29 regarding allelic bias of the Daicel Arbor Prime Plus capture data, we treated different capture data from the same individuals and groups separately, and used them to cross-check the allele sharing analyses. Since the Iranian and South-Central Asian reference samples consisted entirely 1240k or shotgun data, potential artificial affinities between Arbor Prime reference and target data were not expected. Considering the PCA, ADMIXTURE, and D and f3-f4 statistics conducted with our dataset, we conclude that Arbor and Twist captures did not introduce major discrepancies that could affect the interpretation of the results (Figs. 3 and 4, Supplementary Figures S11.4–9, Supplementary Tables S11-12). However, differences were observed in the qpAdm models for these samples (Supplementary Tables S13-14).
On the genomic PCA, we projected ancient samples onto a subset of the modern Eurasian dataset. We observe notable differences among ancient and modern groups inhabiting the areas within and surrounding the ancient Eurasian Silk Road, which also crossed the Iranian Plateau towards the Mediterranean43. Based on principal component 1 (PC1), we identify two distinct clines relevant to our study: (1) the Southern Steppe cline, stretching between Caucasus/Iran and the Central Asian populations19, and (2) the Indus the Periphery Cline, extending between Western Asia and Southern Asia12. Ancient and recent populations of the Iranian Plateau are clustered in the centre and bottom-centre of the PCA plot, along with South Caucasian groups (Fig. 3, Supplementary Figure S11.2).
Eurasian PCA and supervised ADMIXTURE focusing on the genetic variation of Western Asia and its adjacent territories. (A) Grey dots represent modern reference populations, with some labelled in blue. Three-letter codes and detailed information on the ancient and modern populations used in the PCA plot are provided in Supplementary Table S9. A detailed view of the PCA is available in Supplementary Figure S11.2. (B) Supervised ADMIXTURE analyses of the most relevant samples and groups with Iranian EN Ganj Dareh, Anatolian Neolithic (ANF), Onge (AHG), WSHG and Botai (ANE-related), Shamanka Eneolithic (ANA), China LN, and Iron Gates HG (Balkan HG) as fixed sources. For more details and references, see Supplementary Table S10. Individual values and standard errors per component were used for the group-based representation, as described in Methods. Results of the full ADMIXTURE run are presented in Supplementary Figure S11.3.
In the following section, we provide a comprehensive overview of the Iranian prehistoric and historic datasets, highlighting major genetic trends observed through various population genetic methods. The first two principal components position the Iranian Mesolithic and EN samples as distinct from all other ancient and modern samples, indicating their unique base composition, even distinct from CHG, which has been previously discussed as cladal to EN Iran13. Previous studies have described EN groups (Tepe Abdul Hosein and Wezmeh) as homogenous with each other, using either CHG13 or EN Ganj Dareh as unadmixed sources for their modeling12. We consider both EN Ganj Dareh and CHG as suitable local sources for the subsequent populations of the Iranian Plateau. In supervised ADMIXTURE, we observe that CHG harbours the pre-defined Ganj Dareh-related and ANE-related components, aligning with Allentoft et al.15. We note, therefore, that some of the ANE-related component appearing in the Persian Plateau in our analyses may stem from CHG-related ancestry. In subsequent analyses, we tested the potential differences between EN Ganj Dareh and CHG, as well as the impacts of CHG-related components on the Plateau. We conclude that their contributions vary geographically but cannot be separately quantified within the current dataset (f4, qpAdm).
In the same supervised ADMIXTURE analysis, we observe that the ancestry composition of the Natufian and pre-pottery Neolithic individuals from the Levant is represented by 20–25% EN Ganj Dareh and 66–78% ANF ancestries (Supplementary Figure S11.3). This suggests that some Levantine ancestry present on the Persian Plateau might be masked within these components. We took these limitations in consideration during the subsequent analyses.
Genetic structure of the Iranian plateau during the Neolithic-Chalcolithic transition
We studied a new Early Chalcolithic male genome from the 5 th millennium BCE Gol Afshan Tepe site, located on the eastern foothills of the Zagros44, which predates all other published Chalcolithic genomes from Iran5,12. Supervised ADMIXTURE indicates that he was a descendant of Early Neolithic farmers related to the populations of Ganj Dareh, Wezmeh and Tepe Abdul Hossein, all located in the central Zagros area (Fig. 1). On the PCA, the Gol Afshan individual shifts toward more-western ancestry profiles. In f4-statistics, he still shares more genetic drift with EN Ganj Dareh than with CHG, LN Hajji Firuz, Chalcolithic Tepe Hissar or Chalcolithic Seh Gabi (Fig. 4B, S11.5), indicating the EN Iranian component as a more likely source than CHG in this region. Among Neolithic Iranians, the pairwise allele sharing of the Gol Afshan individual with EN Ganj Dareh and Neolithic Tepe Abdul Hosein is not significantly different. Nevertheless, Gol Afshan shares the most alleles with Ganj Dareh and Tepe Abdul Hosein among any other Neolithic and Chalcolithic groups of the Iranian Plateau (Supplementary Figures S11.5). In line with the genetic results, substantial cultural continuity has been detected during the Neolithic-Chalcolithic cultural transition. The Bakun-period Gol Afshan Tepe site was used semi-sedentarily (Supplementary Information, Chap. 1). A ritual ceremony, recorded in the burial type, and the common practice of artificial cranial deformation, also observed at the Gol Afshan Tepe individual, find parallels at the EN Ali Kosh archaeological site in the central Zagros45. Moreover, contemporaneous with Gol Afshan Tepe, numerous instances of artificial cranial deformation and other cultural similarities have been recorded at the Chega Sofla archaeological site in the southern Zagros44,46 (Supplementary Information, Chap. 1). All these findings suggest long-term cultural and biological continuity in and around the Zagros region.
In supervised ADMIXTURE, we observe approximately 8–10% additional AHG component in the Gol Afshan and Tepe Abdul Hosein groups. This minor Neolithic variation, also identified using Tepe Abdul Hosein in the f4-statistics and shown in Fig. 4B-C, suggests the presence of further ancestral pre-Neolithic or Neolithic genetic elements that warrant exploration in future studies. Statistics such as D tests in the form of D(Gol Afshan, Tepe Abdul Hosein, Shahr-i Sokhta BA1/2, Mbuti), comparing between the allele sharing patterns of the two Shahr-i Sokhta genetic groups, suggest that this variation is not a gene flow from the Indo-Iranian borderland, but reflects unascertained genetic diversity within the prehistoric Iranian Plateau. This diversity appears to distinguish most of the eastern regional variation from the northwestern Plateau and the South Caucasus (Supplementary Table S11, Figs. 3 and 4B-C). In subsequent analyses, we demonstrate that several two-way and three-way distal qpAdm models fitting Gol Afshan require AHG as a proxy source. However, Gol Afshan can also be modelled without the AHG-related component (Supplementary Table S13).
Besides the dominance of local EN continuity, the eastward expansion of Levant/ANF-related components is also observable in the mid-5 th millennium BCE Gol Afshan individual44, in addition to previous evidence from northwestern Iranian Late Neolithic-Chalcolithic12,13. This individual exhibits a significant excess of allele sharing with ANF when compared to the Neolithic Iranian genomes from the same Zagros region (see D-statistics in Supplementary Table S11, Z = 4.182). A series of qpAdm tests indicate additional ancestries to the EN Ganj Dareh component in Gol Afshan, such as ANF (modelled with a 17% contributions), or Neolithic Armenia (Masis Blur and Aknashen), LN Hajji Firuz and Chalcolithic Israel, all of which also show significant ANF ancestries12,13. Overall, these show the early admixture events of western influxes into the Neolithic Iranian gene pool. We capture with f4-statistics that every reference group from the Iranian Plateau shares more allele with ANF than with the Natufian and pre-pottery Neolithic Levant (Supplementary Figure S11.9, Supplementary Table S11). We also find that Iraq PPNA exhibits higher allele sharing with ANF than with pre-Chalcolithic Levant or EN Ganj Dareh (Supplementary Figure S11.9). Meanwhile, for the ancient groups of the Iranian Plateau and its surroundings, we demonstrate a correlational increase in allele sharing with the ANF-related ancestries (Turkey_N) and Levant-related ancestries (Jordan_PPNB) (Fig. 4D). These findings indicate the influx of a profile combining both ancestries. This evidence aligns with the commercial interactions of Zagros inhabitants like Ganj Dareh, HajjiFiruz, and Bakun excavation areas with Levantine communities in Syria. These interactions are evidenced by the use of numerical tokens dating back to the Neolithic–Chalcolithic time horizon47. We acknowledge (based on Lazaridis et al.13 and our plausible qpAdm models) that the amount and direction of potential Levantine influx might be characterized better in future research, if additional high coverage comparative data become available.
The correlated allele sharing of the ancient Iranian and surrounding groups shows regional patterns, regardless of temporal differences, with the sharing with ANF and Levant components increasing from east to west (Fig. 4D). Together with the supervised ADMIXTURE analyses, this demonstrates a long-standing East-West genetic cline of the Neolithic Iranian and ANF ancestries13. For instance, this is observable in the northern area through two Chalcolithic sites located more than 700 km apart and from different time periods. The Tepe Hissar group (ca. 3600 − 2000 BCE) exhibits lower ANF-related ancestry (~ 7%) and higher EN Ganj Dareh component compared to a Chalcolithic western Iranian group, such as Seh Gabi (ca. 4800 − 3700 BCE), which carries ~ 27% ANF-related ancestry, as shown in supervised ADMIXTURE and f4-statistics (Fig. 4A). This increase is also observed in the northwest Iranian sites from the LN-BA periods, such as Hajji Firuz, Hasanlu, and Dinkha Tepe, which harbour even higher levels of ANF-related ancestry than Tepe Hissar and Seh Gabi (Fig. 3A-B).
Genetic relations of Chalcolithic and Bronze Age groups of the Persian Plateau
The Mesolithic-EN ancestry forms a predominant basis in the Chalcolithic-Bronze Age genomes from the Iranian Plateau and South-Central Asia. The eastern Iranian BA Shahr-i Sokhta group 1 exhibits a high proportion of EN Ganj Dareh ancestry in its representatives (up to 65% in ADMIXTURE and shown by f4) and an absence of ANF ancestry. The predominant EN Ganj Dareh related ancestry (~ 70% in ADMIXTURE) in the earliest Chalcolithic samples from the Turkmenistan area (Parkhai, Namazga and Geoksyur, dated to ca. 3400 − 2800 BCE) suggests intense connections with the Iranian Plateau. The ANE-related ancestry observed through ADMIXTURE in these genomes aligns with the detection of these components in South-Central Asia by Narasimhan et al.12 and with our f4 statistics (Fig. 4C). Archaeological evidence indicates interactions within the Persian Plateau as early as the EN, through the yet genetically unsampled Jeitun culture’s population, which occupied the territory of modern Turkmenistan48. Published Chalcolithic and BA groups of Turkmenistan (represented here by Geoksyur and Gonur sites) and Uzbekistan (Sapalli Tepe and Bustan sites) exhibit strong genetic similarities with the Chalcolithic Tepe Hissar, BA Shah Tepe, and BA Shahr-i Sokhta group 1 from Iran12,14. The Sapalli culture from Uzbekistan, which thrived from the first half of the 3rd to the mid-2nd millennium BCE, was closely related in material culture to BA Turkmenistan (Namazga, time periods V and VI) and Iran (Shahr-i Sokhta and Hissar III)21, reflecting the observed genetic homogeneity in the region. Meanwhile, ceramic traditions at Shah Tepe and Tepe Hissar demonstrate continuity from the Chalcolithic to BA periods34. Numerous specific artefacts —including soapstone beads, semi-precious stones, and various tools such as local blades, saws, and polished grey pottery—have been discovered at both Shahr-i Sokhta and Tepe Hissar sites, located in the northeastern and eastern Iranian Plateau. Subsequently, these artefacts were widely distributed across sites throughout the eastern and western Persian Plateau, suggesting that these two sites functioned as industrial centres facilitating cross-regional interactions49,50,51. Notably, Shahr-i Sokhta also exhibited increased population diversity, possibly coinciding or correlating with its role (Fig. 3B, see Supplementary Information, Chap. 10).
Stability in the historical periods of the Northern Iranian Plateau
The CHG and EN Iranian ancestries became intermixed by the end of the prehistoric periods, consistent with observations across different periods13,14,52,53,54,55,56. These ancestries persist at levels of around 45–51% in the IA groups of the northwest Iranian Plateau (Hajji Firuz, Hasanlu, Dinkha Tepe sites) in our supervised ADMIXTURE analyses. Furthermore, they remain consistently predominant in the northern Iranian Plateau during the historical period, including the Achaemenid to Sassanid era burial sites of Marsin Chal, Liarsangbon and Vestemin. Most of these individuals can be modelled with CHG as a single source in both individual and group-based qpAdm models (including the combined Iran Historical group). However, the Liarsangbon individuals require an additional western component (16–26% ANF, Neolithic Armenia or Chalcolithic Israel). This finding is further supported by significantly negative f4 results in the form of f4(MersinChal.SG, Liarsangbon.SG, ANF, Mbuti.DG) and aligns resembles the Liarsangbon site’s closer location to the western Iranian Plateau (Supplementary Table S11). Additionally, it also coincides with the cultural differences of Liarsangbon, evidenced by the discovery of non-local artefacts of Egyptian origin from the Roman times (Figs. 3 and 4)57.
Evaluating other possible source populations, we demonstrate through f4-statistics in the form of f4(CHG, Test, Samara_EBA_Yamnaya, Mbuti.DG) and qpAdm models, that the BA Steppe affinities is only apparent due to shared CHG-related ancestries, which were previously defined in the BA Steppe communities52 (represented in our dataset with Samara_EBA_Yamnaya, Supplementary tables S11,13). The AHG-type ancestry detected in ADMIXTURE persists into the historical period. Moreover, in some deep ancestry qpAdm models of the historical individuals, the AHG (Onge) component reaches detectable thresholds.
After assessing the distal ancestries of the historical-period individuals (Marsin Chal, Liarsangbon, Vestemin), we modelled their proximal ancestries using a more focused approach. On the PCA, ADMIXTURE, and f4 analyses (Figs. 3 and 4, Supplementary Figure S11.2–3, S11.6–8), these groups display ancestry patterns similar to those of the northeastern Iranian prehistoric samples, as well as to the BA Gonur and Sapalli Tepe from Turkmenistan and Uzbekistan. In accordance, the qpAdm models indicate a shared genetic ancestry of the historical period samples with the prehistoric northeast Iranians (Chalcolithic Tepe Hissar, BA Shah Tepe) or South-Central Asians (represented with Chalcolithic Geoksyur and BA Gonur group 1). Our analyses reveal that everyone out of the seven historical-period individuals yielding sufficient genome-wide data can be modelled either with one of these prehistoric groups as a single source or in a two-way model with an additional western Iranian source. In group-based analyses, shotgun genomes, such as those of the Marsin Chal and Liarsangbon groups (n = 2 in each) can also be modelled with the same sources. However, the proportions of the northwestern IA components with elevated ANF/Levant-related ancestries are more prominent in Liarsangbon (Supplementary Table S14). IA Hajji Firuz is the only additional western source that provides plausible models for all historical groups. Using this source, Marsin Chal can be modelled with ~ 78% BA Shah Tepe and additional ~ 22% IA Hajji Firuz, while for the Liarsangbon group these amounts are ~ 37% BA Shah Tepe and ~ 63% IA Hajji Firuz. The combined group, referred to as Iran_North_Historical, produces similar results and can be modelled with ~ 52% BA Shah Tepe and ~ 48% IA Hajji Firuz-type contributions (Supplementary Table S14). We interpret these findings as evidence that the genetic profiles of historical-period groups of the northern Iranian Plateau reflect their position along the broader east-west genetic cline, rather than resulting from specific admixture events.
Scattered evidence on medieval and modern Iranian populations
The Medieval period individual from the Kalmakareh Cave in western Iran has a genetic profile similar to those of the northwestern groups of the Iranian Plateau, such as Hasanlu and Hajji Firuz, as well as a Medieval sample from Ganj Dareh, as observed both on the PCA and in ADMIXTURE. This, combined with f4 and distal qpAdm results, indicates a reduction of Iran Neolithic ancestry in the western Iranian Plateau over time. Meanwhile, most of the genetic ancestry of the newly published individual can be modelled in qpAdm with the mentioned IA variety in the Plateau, such as IA Hajji Firuz as the single source or as a mixture of IA Hasanlu (~ 80%) and another source (e.g., ~ 20% Shah Tepe from the northeast). The lack of published ancient individuals from southwestern Iran limits further investigation into the regional genetic ancestries.
On the PCA, modern Iranians align with the majority of the Iranian prehistoric and historic genetic variation, but do not exhibit the Indus Periphery Cline characteristics previously described in the BA by Narasimhan et al.12. In addition to clustering together in the PCA, the Mazandarani and Fars groups of Iran also show similar population models, albeit in different proportions, in the qpAdm analyses performed on the Human Origin dataset (Supplementary Table S14). Further Medieval and modern genome-wide sample pools are required to better understand the formation of the modern Iranian genetic variation.
Two-dimensional scatterplots of seven f4-statistics. The four panels represent scatterplots of different f4 model combinations. f4 estimates and corresponding Z-scores are provided in Supplementary Table S11. Non-significant Z-scores are indicated with red error bars. Blue lines represent regression analyses (Weighted Least Squares (WLS) linear regression) and blue stars denote groups that deviate significantly from the regression, based on the model fit and the variability captured by the weighted standard deviation. Data produced using different laboratory methods from the same sample is indicated with a single symbol.
Conclusions
In this study, we presented novel ancient mitogenomes and genome-wide data from previously unstudied areas of the Iranian Plateau, with a particular focus on the northern area, southward of the Caspian Sea. We revealed several insights into the genetic history of the ancient Iranian populations and provided a comprehensive overview of the available ancient DNA data from Western Asia.
We explored the influence of major ancestry sources on the new dataset. We reproduced previous results on a prehistoric East-West cline of the EN Iranian ancestry with Neolithic Anatolian (ANF) and Levant-related autosomal ancestries. This cline exerted a lasting imprint on the population of the Iranian Plateau up to the historical period. The allele sharing with both ANF and Neolithic Levant increased towards the western end of this cline. We also discussed varying dual ancestry patterns of CHG and EN Ganj Dareh ancestries in ancient peoples of the Plateau. Furthermore, we found signals for a previously undescribed (AHG-like) ancestry in the Iranian Neolithic farmers that likely distinguished the Iranian Plateau’s population from more westerly groups, such as the contemporaneous South Caucasians. These observations indicate long-term genetic tendencies in the Iranian Plateau.
The new Early Chalcolithic genome from southwestern Iran presented in this study showed closer alignment with Early Neolithic Iranian farmers, with additional contributions from other Neolithic groups in western and northwestern proximities. This finding suggests predominant continuity, but also that the western Iranian region maintained contact with neighbouring areas, facilitating the introduction of western ancestries into the Iranian Plateau during the early stages of the Neolithic-Chalcolithic transition.
We demonstrated a strong Iranian Neolithic and CHG substrate in the historical-period samples from northern Iran, where these genetic components persisted in the pre-Medieval era. We confirmed the continuity from the Chalcolithic-Bronze Age into this period in northeastern Iran, despite this area hosting part of the Silk Road, which facilitated extensive human movement. Bronze Age Steppe ancestry remained relatively minor during the historical period in northern Iran. Instead, the historic period population of the northern Iranian Plateau exhibited strong genetic affinities with the Chalcolithic and Bronze Age communities of Turkmenistan, and northeastern-eastern Iran, forming homogeneous groups in our analyses as a part of the described Southwest Asian cline. As only one Iron Age genome is available from Turkmenistan, and there are none from the northeastern Iranian Plateau, further sampling is necessary to investigate the dynamics of this era, particularly to determine whether contacts between the two regions were sustained or disrupted after the Bronze Age.
Although only two Medieval genomes are currently available from the Iranian Plateau (one published in this study), the data indicate that the majority of the ancient Iranian gene pool remained stable over the centuries, with minor changes observed in the contemporary Iranian population. This suggests enduring genetic variation over millennia. Notably, at least half of the genetic heritage in Medieval southwestern Iran originated from Neolithic Iranian farmers and likely transmitted through Iron Age Iranians, despite the region’s exposure to external interactions with Mesopotamia, the Levant, the Caucasus and Anatolia. This highlights the genetic stability of the region’s inhabitants, even in the face of historical migrations and cultural shifts.
In the newly published dataset, we described Y-chromosomal and mitochondrial haplogroups that evolved around the ancient Persian Plateau, and which are still rare in ancient genome databases. We compared these with the available ancient and modern data and showed the long-term continuity in the uniparental ancestries in the region.
In summary, this research provides new evidence enhancing our understanding of the genetic characteristics and connections of ancient Iranian populations, while further comprehensive sampling is still required to uncover their internal diversity.
Materials and methods
Sampling strategy
In our genetic analyses, we examined samples from a variety of archaeological sites across Iran, representing a wide range of periods and cultures. We collected a total of 50 samples from nine different archaeological sites. From the earliest periods, we sampled from the Early Chalcolithic culture (Middle Bakun period), which is represented by the Gol Afshan Tepe site and located in the southern Zagros Mountains44. The Late Chalcolithic through to the Late Bronze Age are represented in our dataset by samples from Gohar Tepe in the northern Iranian Mazandaran, Shahr-i Sokhta in southeastern Iranian Sistan and Baluchestan Province, and Cham Papi archaeological site in the southwestern Ilam Province of modern Iran. Details on these sites can be found in the Supplementary Information.
The Iron Age Neo-Elamite period (1100 − 539 BCE) is represented by samples from the Jubaji burial tomb in Khuzestan Province. Samples from the historical period originate from the Achaemenid to Seleucid periods (355 − 280 BCE) at Marsin Chal in Semnan Province. From the Parthian Empire (247 BCE-224 CE), the samples were collected from Liarsangbon in Gilan Province. The late Parthian to Middle Sassanid Empire periods (200–460 CE) are represented by samples from Vestemin in Mazandaran Province, all from northern Iran. Lastly, the Medieval Period (1215–1270 CE) is represented by samples from Kalmakareh cave in Lorestan Province (Supplementary Table S1, Fig. 1).
Sampling
For genetic investigation, we prioritised sampling petrous bones if they were available, due to their favourable DNA preservation properties58. Alternatively, we collected teeth and long bones59.
Radiocarbon dating
Radiocarbon dating was performed at the HUN-REN ATOMKI AMS C-14 facility of the Isotope Climatology and Environmental Research Centre in Debrecen, Hungary. Before Present (BP) dates were calibrated in Oxcal software v4.4, using IntCal 20 calibration curve60,61 (Supplementary Table S1).
Ancient sample Preparation and DNA extraction
All steps of sampling, DNA extraction, and library preparation were carried out in the dedicated ancient DNA facility of the HUN-REN RCH Institute of Archaeogenomics. After thorough mechanical surface cleaning and UV-treatment, we drilled the samples to gain powder from them62, with the exception of four samples that were ground in a mixer mill. An additional three samples were processed with the minimally destructive protocol developed by Harney et al.63. DNA was extracted from 50 mg bone powder either manually64or using a liquid-handling automated workstation65. As for the three minimally destroyed samples, 150 µl lysate was used for DNA extraction. Three very short fragments of the mitochondrial DNA (mtDNA) were amplified with primers from the GenoCoRe22 assay66 in order to verify the success of DNA extraction.
DNA library preparation, hybridisation captures and sequencing
We prepared half-UDG treated, double-stranded libraries with unique barcoded adapters following well-established protocol, incorporating minor changes for automation67. Libraries were amplified with TwistAmp Basic (Twist DX Ltd), followed by purification with AMPure XP beads (Agilent). Finally, unique indexes were added to each library in a PCR reaction for multiplex sequencing68.
In some cases, we carried out hybridization capture to selectively enrich specific targeted regions of the genome. Mitochondrial genomes were captured as described in Csáky et al.69, while genomic variants were detected with myBaits Arbor Expert Human Affinities “Complete” Kit (Daicel Arbor Biosciences, which includes the ancestral 850 K SNPs set in addition to the ones from “Prime Plus” (1240k, Y, and mitochondrial SNPs). After concerns were raised about the Prime Plus portion of the Arbor capture29, we repeated the most important samples with Ancient Human DNA Panel Workflow (Twist Bioscience)30.
Samples that passed the screening sequencing on an Illumina MiSeq system with Reagent Kit v3 (2 × 75 cycles) were later submitted to Novogene Ltd for deep sequencing on NovaSeq 6000 platform, using 2 × 150 cycles.
Criteria for deeper shotgun sequencing were > 40% endogenous DNA content and < 2% duplicated reads mapped on the genome. Criteria for genomic capture were 4–40% endogenous DNA detected and < 5% duplicated reads at the shallow shotgun screening (Supplementary Table S2).
Bioinformatic analysis of the NGS data
We used the same in-house pipeline for raw sequence data processing as described in Gerber et al.70, the PAPline (https://github.com/ArchGenIn/papline) (for Supplementary Table S1). The ancient data as BAM and FASTQ files were uploaded to ENA, under the project number PRJEB81975 (https://www.ebi.ac.uk/ena/browser/home). The GRCH37.p13 reference sequence was used to call the pseudohaploid genomes.
We used BREADR package (Biological Relatedness from Ancient DNA in R) to assess kinship among the samples32, for results, see (Supplementary Table S8). Haplogroup determination for mtDNA was performed by HaploGrep 2 on FASTA files that were called with a custom R script tailored for archaic DNA71. Using the deep-sequenced genomes, we determined the Y-chromosomal haplogroups with Yleaf v1 software72.
Contamination levels of the mtDNA were estimated using the ContamMix software73 X-chromosomal contamination in male individuals was measured with two software tools: ANGSD (version 0.939–10-g21ed01c, htslib 1.14–9-ge769401) and hapCon (hapROH package version 0.60)74. The doCounts for the former method was run on X-chromosomal region from positions 5,500,000 to 154,000,000, with base quality 30 and mapping quality 25. The toolkit’s contamination executable was used to process the resulting file. Parameters were set to -b 5,500,000 -c 154,000,000 -d 3 -e 100 -p 1. Additionally, the file specified by the -h parameter was included as provided by the software developers. For results, see Supplementary Table S3 and S4.
Runs of homozygosity were calculated with the hapROH program33, run with default parameters for all pseudo-haploid genotypes with at least 150k SNP covered from the 1240k SNP panel, and repeated for the merged BAM files including libraries captured via the two above-detailed methods (Supplementary Table S13).
Population genetic analyses
We performed various population genetic analyses, in which we compared the studied populations to several other ancient and modern-day published datasets. We compiled AADR v54.139 with further relevant studies from Western and Central Asia14,56,75,76,77,78,79,80,81,82.
CHG group was created by combining KK1_noUDG.SG and SATP_noUDG.SG83. EHG group was created with a combination of I0061, I0211, I0124, Sidelkino_noUDG.SG28,84. China_LN was prepared as a combination of the China_Upper_YR_LN and China_YR_LN groups85. Levant_preChalcolithic included Jordan_PPNB and Israel_Natufian13. Armenia_N was a combination of I1390 and I139113. West_Siberia_N_Botai was a composite group including the individuals BOT14, BOT2016, I1960, I576612,19.
The Principal Component Analysis (PCA) was computed using the EIGENSOFT smartpca software (v16000)86 with the Human Origins Panel SNP set39 and a reduced set of West-Central Eurasian modern populations for calculation, as listed in Table S9. For every other analysis, the 1240k array SNP set28 was used for variant calling. To estimate ancestry composition, first we used supervised ADMIXTURE analysis (K = 7) calculated by the ADMIXTURE v1.3.0 software42. The seven fixed ADMIXTURE source components were chosen depending on their previously described genetic impacts on the Eurasian continent (details of the sources are in Table S10)12,19,66,85,87,88. The standard error bars of the components in Fig. 3B, were calculated using point estimation and bootstrapping, as described in the official ADMIXTURE manual. We then calculated mean component values for the groups and the group standard errors with the formula= (√(SE1²+SE2²+.+SEn²))/n, where “n” represents the number of samples in each analysis group. f-statistics and qpAdm were performed using AdmixTools v7.0.140 and ADMIXTOOLS v241.
Data analysis and visualization of the f-statistics was performed in Python using pandas (data handling), numpy (numerical operations), matplotlib (visualization), and statsmodels (regression analysis). Weights were calculated as the inverse of the combined variances to account for measurement precision. Weighted Least Squares (WLS) regression was used to model the relationships between f4 variables, with weights assigned inversely to measurement variance, prioritizing more precise data points. A constant was added to the predictor variable to include an intercept. Slope and intercept were extracted, and residuals were calculated to evaluate prediction accuracy. Outliers were identified on the f4 plots based on a weighted standard deviation of residuals, with a threshold set at twice this standard deviation. Points exceeding this threshold were flagged as significant deviations. Scatter plots with error bars, color-coded by Z-scores, were created to visualise each comparison and highlight outliers.
To elucidate the ancestries on a global and regional bases, we tested deep and proximal ancestry qpAdm models for the newly analysed dataset, where we considered only the individuals with at least 100k SNP covered on the 1240k panel. For the distal qpAdm models, the right group set consisted of Mbuti.DG, Papuan.DG, China_LN, Luxembourg_Loschbour.DG, Russia_MA1_HG.SG, EHG, Morocco_Iberomaurusian, Turkey_Epipaleolithic, Levant_preChalcolithic. In the proximal qpAdm models, we used Mbuti.DG, ONG.SG (Onge), Caucasian HG (CHG), EHG, Turkey_N, Jordan_PPNB, Russia_MA1_HG and Serbia_IronGates_Mesolithic as right groups. These populations in the two right group sets have diverse genetic relationships to the ancient populations of the Iranian Plateau and its surroundings, as well as to the tested left group sets, which are expected to enhance the resolution of qpAdm analyses89. We refrained from rotating the left and right group populations, due to severe criticism raised in recent research90. For the qpAdm analyses we used the qpadm_multi function of ADMIXTOOLS v2 with the parameter allsnps: NO (as allsnps = FALSE as an argument of the qpadm_multi function). Group-based proximal qpAdm models were run for the historical-period Liarsangbon and Marsin Chal shotgun genomes. In addition, the Iran_North_Historical group was created using these individuals and the historical period Vestemin individual. Our in-house script for qpAdm analyses considered only the results with ≤ 0.2 (20%) standard error per source component in the model. In addition, results with standard error higher than the source proportion, or those with a negative value for at least one source proportion were deemed unfeasible. Feasible models in distal analyses are presented in Table S13. Unfeasible models in proximal analyses are presented with p-value = 0 (no decimals) and NA in source proportion columns (Supplementary Table S14). When evaluating qpAdm models, we accounted for high false-positive rates reported recently90, and chose the most likely models based on the PCA, ADMIXTURE and f-statistics. Between two-way and three-way models, we preferred the lower-ranking ones.
Data availability
The ancient human DNA sequence data presented in this study are openly available in the European Nucleotide Archive at https://www.ebi.ac.uk/ena/browser/search upon publication, with accession numbers PRJEB81975, ERP165740.
Abbreviations
- AHG:
-
Andamanese Hunter-Gatherer
- ANE:
-
Ancient North Eurasian
- ANF:
-
Anatolian Neolithic Farmer
- BA:
-
Bronze Age
- C:
-
Chalcolithic
- EN:
-
Early Neolithic
- LN:
-
Late Neolithic
- H:
-
Historical period
- HG:
-
Hunter-Gatherer
- IA:
-
Iron Age
- mtDNA:
-
mitochondrial DNA
- N:
-
Neolithic
- PCA:
-
Principal Component Analysis
- WSHG:
-
Western Siberian Hunter-Gatherer
References
Vallini, L. et al. The Persian plateau served as hub for Homo sapiens after the main out of Africa dispersal. Nat. Commun. 15, 1882 (2024).
Rante, R. Khorasan proper and greater Khorasan within a politico-cultural framework. Gt Khorasan Hist. Geogr. Archaeol. Mater. Cult. Stud. Hist. Cult. Middle East. 29, 9–26 (2015).
Biscione, R. & Vahdati, A. A. The BMAC presence in Eastern Iran: state of affairs in December 2018–towards the greater Khorasan civilization? in The World of the Oxus Civilization 527–550 Routledge (2020).
Guran, S. H., Yousefi, M., Kafash, A. & Ghasidian, E. Reconstructing contact and a potential interbreeding geographical zone between neanderthals and anatomically modern humans. Sci. Rep. 14, 20475 (2024).
Lazaridis, I. et al. Genomic insights into the origin of farming in the ancient Near East. Nature 536, 419–424 (2016).
Matthews, R. & Fazeli Nashli H. The Archaeology of Iran from the Palaeolithic to the Achaemenid Empire. Routledge (2022).
Broushaki, F. et al. Early Neolithic genomes from the eastern Fertile Crescent. Science (80-). 353, 499–503 (2016).
Gallego-Llorente, M. et al. The genetics of an early Neolithic pastoralist from the Zagros, Iran. Sci. Rep. 6, 31326 (2016).
Vahdatinasab, H. et al. Dispersals of the Aryans into the Iranian Plateau in the light of new genetic discoveries, case study. Iran. J. Anthropol. Res. 7, 117–143 (2017).
Ramezani, M. et al. Sequencing mitochondrial DNA of Middle Elamite skeletal remains from Haft Tepe. J. Archaeol. Stud. 9, 73–86 (2017).
Saadatmand, F. Extraction and Sequencing of Mitochondrial DNA (mtDNA) of Human Skeletal Remains of Iron Age 2 Qara Tepe Segezabad. Islamic Azad University (2019).
Narasimhan, V. M. et al. The formation of human populations in South and Central Asia. Science 365, (2019).
Lazaridis, I. et al. The genetic history of the Southern Arc: A bridge between West Asia and Europe. Science (80-.). 377, (2022).
Koptekin, D. et al. Spatial and Temporal heterogeneity in human mobility patterns in holocene Southwest Asia and the East Mediterranean. Curr. Biol. 33, 41–57e15 (2023).
Allentoft, M. E. et al. Population genomics of post-glacial Western Eurasia. Nature 625, 301–311 (2024).
Shinde, V. et al. An ancient Harappan genome lacks ancestry from steppe pastoralists or Iranian farmers. Cell 179, 729–735e10 (2019).
Saadatmand, F. et al. Introducing the human mitochondrial DNA haplogroup from Sagzabad, Qazvin, Iran. Mod. Genet. 19, 173–182 (2024).
Moradi, H. Excavations in square X in the ERA of Shahr-i Sokhta: pottery traditions and cultural interaction during period I (ca. 3550 – 3000 BC). Excavations Researches Shahr-i Sokhta. 2, 143–182 (2023).
Jeong, C. et al. The genetic history of admixture across Inner Eurasia. Nat. Ecol. Evol. 3, 966–976 (2019).
Niknami, K. A. & Hozhabri, A. Archaeology of Iran in the Historical Period (Springer, 2020).
Sajjadi, S. Commercial and cultural relations of Sistan with neighboring areas during the 3rd millennium BC. Anc. Iran. Stud. 2, 3–34 (2023).
Waters, M. Ancient Persia: A Concise History of the Achaemenid Empire, 550–330 BCE (Cambridge University Press, 2014).
Choksy, J. K. & Josef Wiesehöfer. Ancient Persia: From 550 BC to 650 AD. Translated by Azizeh Azodi. London: IB Tauris, distributed by St. Martin’s, New York. Pp. xiv, 329. (2000). (1998).
Salamon, M. & Józef Wolski Expert in Parthian history, Historian of Antiquity. Palamedes A J. Anc. Hist. 9–16 (2008). (1920)–2008.
Eskandari, N., Pfälzner, P. & Soleimani, N. A. The formation of the Early Bronze Age Jiroft culture, Halil Rud basin, southeastern Iran: excavations at Varamin/Jiroft 2017. Anc. Near East. Stud. 60, 5–91 (2023).
Sajjadi, S. Catacomb graves or burial sites of clan chiefs of the 3rd millennium BC. Shahr-i Sokhta confederation. Zbrika Naubouykh Pr Movata Istor Vyp 383 Akad. Nauk. Vyshchoi Ovity Ukr. (2015).
Frankopan, P. The Silk Roads: A New History of the World. London: Bloomsbury Publishing. (2015)
Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503 (2015).
Davidson, R. et al. Allelic bias when performing in-solution enrichment of ancient human DNA. Mol. Ecol. Resour. 23, 1823–1840 (2023).
Rohland, N. et al. Three assays for in-solution enrichment of ancient human DNA at more than a million SNPs. Genome Res. 32, 2068–2078 (2022).
Tree, Y. D. H. International Society of Genetic Genealogy; at (2019). (2020).
Rohrlach, A. B., Tuke, J., Popli, D. & Haak, W. BREADR: An R package for the Bayesian estimation of genetic relatedness from low-coverage genotype data. bioRxiv 2023.04.17.537144 (2023). https://doi.org/10.1101/2023.04.17.537144
Ringbauer, H., Novembre, J. & Steinrücken, M. Parental relatedness through time revealed by runs of homozygosity in ancient DNA. Nat. Commun. 12, 5425 (2021).
Orsaria, F. Shah Tepe: a new approach to an old excavation. Riv Studi Orient. 69, 481–495 (1995).
Amjadi, M., Hayatmehr, Z., Egyed, B. & Tavallaei, M. Szécsényi-Nagy, A. A comprehensive review of HVS‐I mitochondrial DNA variation of 19 Iranian populations. Ann. Hum. Genet. 88, 259–277 (2024).
Garthwaite, G. R. Tribes, Confederation and the State: an historical overview of the Bakhtiari and Iran. in Tribe and State in Iran and Afghanistan (RLE Iran D) 314–330. Routledge, (2012).
Sahakyan, H. et al. Origin and diffusion of human Y chromosome haplogroup J1-M267. Sci. Rep. Mar 23;11(1):6659. (2021).
Singh, S. et al. Dissecting the influence of Neolithic demic diffusion on Indian Y-chromosome pool through J2-M172 haplogroup. Sci. Rep. 6, 19157 (2016).
Mallick, S. et al. The Allen ancient DNA resource (AADR) a curated compendium of ancient human genomes. Sci. Data. 11, 182 (2024).
Patterson, N. et al. Ancient admixture in human history. Genetics 192, 1065–1093. (2012).
Maier, R. et al. On the limits of fitting complex models of population history to f-statistics. eLife 12 (e85492). https://doi.org/10.7554/eLife.85492 (2023).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Hansen, V. The Silk Road (Oxford University Press, 2012).
Taheri, M. H. Regional settlement system and social-cultural development of communities in the North of Fars (Semirom county) During the Bakun period based on Gol-Afshan site excavation. PhD Thesis University of Tehran. (2022).
Niknami, K. A., Ramazani, M. & Niknami, N. Ali Kosh lady and her artificially modified head: an appraisal. Iran. J. Archaeol. Stud. 1, 17–24 (2011).
Alirezazadeh, M., Vahdatinasab, H. & Moghaddam, A. Modification of human skulls in Tol-e Chega Sofla. pazhoheshha-ye Bastan Shenasi Iran. 14, 7–33 (2024).
Niknami, K. A., Taheri, M. H. & Sardary, A. Evidence for an early accounting system found at Tal-e Mash Karim, a Chalcolithic site in Iran. Doc. Praehist. 45, 100–107 (2018).
Roustaei, K. & Nokandeh, J. Neolithic developments in the Gorgan plain, south-east of the Caspian Sea. Antiquity 91, e3 (2017).
Fisher, M. T. The New Chronology of the Bronze Age Settlement of Tepe Hissar, Iran. Classical Presences Series. (2018).
Moradi, H. Architectural Style & Technique in Prehistoric Sistan. Sustainable patterns in Shahr-i-Sokhta architecture from early 3rd millennium to the beginning of the 2nd millennium BC. Ash-sharq Bull. Anc. Near East–Archaeological Hist. Soc. Stud. 4, 57–89 (2020).
Moradi, H. & Rajabioun, Z. Socio–Economic analysis of Shahr-i Sokhta period I-IV (early to late 3rd millennium BC) based on statistical analysis of goods and structures of the graves at necropolis. J. Archaeol. Stud. 107–129 (2022).
Wang, C. C. et al. Ancient human genome-wide data from a 3000-year interval in the Caucasus corresponds with eco-geographic regions. Nat. Commun. 10, 590 (2019).
Feldman, M. et al. Ancient DNA sheds light on the genetic origins of early Iron Age Philistines. Sci. Adv. 5, (2019).
Skourtanioti, E. et al. Genomic history of Neolithic to Bronze Age Anatolia, Northern Levant, and Southern Caucasus. Cell 181, 1158–1175e28 (2020).
Altınışık, N. E. et al. A genomic snapshot of demographic and cultural dynamism in upper Mesopotamia during the Neolithic transition. Sci. Adv. 8, eabo3609 (2022).
Guarino-Vignon, P. et al. Genome-wide analysis of a collective grave from Mentesh Tepe provides insight into the population structure of early Neolithic population in the South Caucasus. Commun. Biol. 6, 319 (2023).
Jahani, V. et al. Typology, and relative chronology of personal ornaments from the ancient cemetery of Liār-Sang-Bon, Amlash, Iran. Persica Antiq. 3, 37–49 (2023).
Pinhasi, R. et al. Optimal ancient DNA yields from the inner ear part of the human petrous bone. PLoS ONE. 10 (6), e0129102. https://doi.org/10.1371/journal.pone.0129102 (2015).
Parker, C. et al. A systematic investigation of human DNA preservation in medieval skeletons. Sci. Rep. 10, 18225 (2020).
Ramsey, C. B. & Lee, S. Recent and planned developments of the program OxCal. Radiocarbon 55, 720–730 (2013).
Reimer, P. J. et al. The IntCal20 Northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020).
Sirak, K. A. et al. A Minimally-Invasive method for sampling human petrous bones from the cranial base for ancient DNA analysis. Biotechniques 62, 283–289 (2017).
Harney, É. et al. A minimally destructive protocol for DNA extraction from ancient teeth. Genome Res. 31, 472–483 (2021).
Dabney, J. & Meyer, M. Extraction of highly degraded DNA from ancient bones and teeth. Methods Mol Biol. 2019:1963:25-29. doi: 10.1007/978-1-4939-9176-1_4
Rohland, N., Glocke, I., Aximu-Petri, A. & Meyer, M. Extraction of highly degraded DNA from ancient bones, teeth and sediments for high-throughput sequencing. Nat. Protoc. 13, 2447–2461 (2018).
Haak, W. et al. Ancient DNA from European early Neolithic farmers reveals their Near Eastern affinities. PLoS Biol. 8, e1000536 (2010).
Rohland, N., Harney, E., Mallick, S., Nordenfelt, S. & Reich, D. Partial uracil–DNA–glycosylase treatment for screening of ancient DNA. Philos. Trans. R Soc. B Biol. Sci. 370, 20130624 (2015).
Meyer, M. & Kircher, M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. pdb.prot5448 (2010). (2010).
Csáky, V. et al. Genetic insights into the social organisation of the Avar period elite in the 7th century AD Carpathian basin. Sci. Rep. 10, 948 (2020).
Gerber, D. et al. Interdisciplinary analyses of Bronze Age communities from Western Hungary reveal complex population histories. Mol. Biol. Evol. 40, msad182 (2023).
Weissensteiner, H. et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 44, W58–W63 (2016).
Ralf, A., Montiel González, D., Zhong, K. & Kayser, M. Yleaf: Software for human Y-Chromosomal haplogroup inference from Next-Generation sequencing data. Mol. Biol. Evol. 35, 1291–1294 (2018).
Fu, Q. et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr. Biol. 23, 553–559 (2013).
Huang, Y. & Ringbauer, H. HapCon: estimating contamination of ancient genomes by copying from reference haplotypes. Bioinformatics 38, 3768–3777 (2022).
Kumar, V. et al. Genetic continuity of Bronze Age ancestry with increased steppe-related ancestry in late Iron Age Uzbekistan. Mol. Biol. Evol. 38, 4908–4917 (2021).
Guarino-Vignon, P. et al. Genetic analysis of a Bronze Age individual from Ulug-depe (Turkmenistan). Front. Genet. 13:884612. (2022) doi: 10.3389/fgene.2022.884612
Skourtanioti, E. et al. Ancient DNA reveals admixture history and endogamy in the prehistoric Aegean. Nat. Ecol. Evol. 7, 290–303 (2023).
Kumar, L. et al. Genetic Affinities and Adaptation of the South-West Coast Populations of India. Genome Biol. Evol. 15, evad225 (2023).
Mattila, T. M. et al. Genetic continuity, isolation, and gene flow in Stone Age Central and Eastern Europe. Commun. Biol. 6, 793 (2023).
Kumar, V. et al. Bronze and Iron Age population movements underlie Xinjiang population history. Scence (80-). 376, 62–69 (2022).
Wang, H. et al. Human genetic history on the Tibetan Plateau in the past 5100 years. Sci. Adv. 9, eadd5582 (2023).
Sirak, K. et al. Medieval DNA from Soqotra points to Eurasian origins of an isolated population at the crossroads of Africa and Arabia. Nat. Ecol. Evol. 8, 817–829 (2024).
Jones, E. R. et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun. 6, 8912 (2015).
de Damgaard, B. The first horse herders and the impact of Early Bronze Age steppe expansions into Asia. Science (80-). 360, eaar7711 (2018).
Ning, C. et al. Ancient genomes from Northern China suggest links between subsistence changes and human migration. Nat. Commun. 11, 2700 (2020).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Järve, M. et al. Shifts in the genetic landscape of the Western Eurasian steppe associated with the beginning and end of the Scythian dominance. Curr. Biol. 29, 2430–2441e10 (2019).
Mondal, M. et al. Genomic analysis of Andamanese provides insights into ancient human migration into Asia and adaptation. Nat. Genet. 48, 1066–1070 (2016).
Harney, É., Patterson, N., Reich, D. & Wakeley, J. Assessing the performance of QpAdm: a statistical tool for studying population admixture. Genetics 217, iyaa045 (2021).
Flegontova, O. et al. Performance of qpAdm-based screens for genetic admixture on admixture-graph-shaped histories and stepping-stone landscapes. bioRxiv 2023.04.25.538339; (2023). https://doi.org/10.1101/2023.04.25.538339
Acknowledgements
We thank Krisztián Oross for his helpful comments on radiocarbon dating. Our sincere appreciation is extended to Dr. Seyed Mansur Seyed Sajjadi, Dr. Arman Shishegar and Dr. Lili Niakan from the Iran Center for Archaeological Research (ICAR), Dr. Ali Mahfroozi and Serollah Ghasemi from the Mazandaran Research Institute for Cultural Heritage and Tourism, Ms. Somayeh Chavoshi from the Noor Human Genetic Research Center and Ms. Beheshteh Nasiri Rad from Ministry of Cultural Heritage, Tourism and Handicrafts for generously providing samples for this research. We express special thanks to Dr. Jebrael Nokandeh, Director of the National Museum of Iran, and Mr. Javad Nasiri for accessing valuable museum collections. Moreover, we thank Mr. Rohollah Zarin Pour from the Falak-ol-Aflak Museum for accessing valuable museum collections. We extend our gratitude to Prof. Kamalodin Niknami and Prof. Hassan Karimian from Tehran University for their expertise in Iranian Archaeological contexts and Mr. Farzad Frouzanfar from the ICAR for his insightful guidance throughout the anthropological endeavor. We extend our appreciation to Ms. Parastoo Erfanmanesh and Mr. Jalil Golshan from the Research Institute of Cultural Heritage & Tourism. Their tireless advocacy and outreach efforts were crucial in raising awareness about this type of research. We also extend our appreciation to Miss Forough Taheri from the Metabolic Disorders Research Centre, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Dr. Raofian and Dr. Danesh from the Legal Medicine Organization of Iran, Mr. Alireza Abolfazli, Director of ARA Electronic Company, Mr. N. Atighech from the Student Affairs Organization of Iran, Ms. Masoumeh Heidarian, Ms. Norasteh Abolfazli and Mr. Hamid Abolfazli from the Cultural Heritage, Ms. Masoumeh Sabri from the Iranian Red Crescent Society, Mr. Sadegh Taheri PhD Student, Department of Animal Science, Ferdowsi University of Mashhad, Dr. Sepideh Maziar from Institute of Archaeological Sciences, Dept. of Near Eastern Archaeology Goethe University, Frankfurt and Mr. Mehdi Amjadi for their support. We are thankful to the Research Institute Of Cultural Heritage and Tourism and the National Archaeological Research Institute for facilitating the acquisition of the National Legal Permits to work on ancient skeletal human remains.
Funding
Open access funding provided by Eötvös Loránd University.
This research paper was funded by the HUN-REN Research Centre for the Humanities. MAA was supported by the Tempus Foundation and the UNESCO Silk Roads Programme and the National Commission of the People’s Republic of China for UNESCO. ASN was supported by the MTA-BTK Lendület ‘Momentum’ Bioarchaeology Research Project.
Author information
Authors and Affiliations
Contributions
Conceptualization: MAA, MR and ASN; Methodology: MAA, MR, ASN and BGM; Collecting the samples: MAA, MR, AB, ZS, MHT, PZ, AH, VJ, AMS, and MT; Performed the DNA extractions and library preparation: MAA and MM; Conducted the bioinformatics analyses: MAA, YCO, KJ, ZH, and ASN; Performed the statistical analyses and data interpretation: MAA, YCO, MR, and ASN; Resources: MAA, MR, AB, MHT, HM, PZ, AH, VJ, AMS, BE, MT and ASN; Visualization: MAA and ASN; Writing – original main draft of the paper: MAA, YCO, MR, and ASN; Writing – review & editing: MAA, YCO, MR, and ASN; Funding acquisition: BGM, MR, MT, and ASN; Supervision: MR, MT and ASN.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
Ancient samples were taken with the written consent of the stakeholder museums and the excavator and processor scholars. Human remains were treated with dignity and respect, to ensure minimal destruction of the samples.
Conflict of interest
The authors have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, and interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amjadi, M.A., Özdemir, Y.C., Ramezani, M. et al. Ancient DNA indicates 3,000 years of genetic continuity in the Northern Iranian Plateau, from the Copper Age to the Sassanid Empire. Sci Rep 15, 16530 (2025). https://doi.org/10.1038/s41598-025-99743-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99743-w