Abstract
Endovascular treatment (EVT) for vertebrobasilar artery occlusion (VBAO) with atrial fibrillation presents complex clinical challenges. This comprehensive multicenter study of 525 patients across 15 Chinese provinces investigated nuanced predictors beyond conventional metrics. While 45.1% achieved favorable outcomes at 90 days, our advanced machine learning approach unveiled subtle interaction effects among clinical variables not captured by traditional statistical methods. The predictive model distinguished high-risk subgroups by integrating multiple parameters, demonstrating superior prognostic precision compared to standard NIHSS-based assessments. Novel findings include nonlinear relationships between dyslipidemia, stroke severity, and functional recovery. The developed predictive algorithm (AUC 0.719 internally, 0.684 externally) offers a more sophisticated risk stratification tool, potentially guiding personalized treatment strategies in high-complexity VBAO patients with atrial fibrillation.
Similar content being viewed by others
Introduction
Acute vertebrobasilar artery occlusion (AVBAO) is a severe form of ischemic stroke, accounting for approximately 1% of all ischemic strokes and 5–10% of proximal intracranial occlusions1,2. Despite advancements in stroke management, AVBAO is associated with mortality and severe disability rates of up to 80%2. While mechanical thrombectomy (MT) is standard care for anterior circulation large vessel occlusions, its efficacy in AVBAO remains controversial. Recent randomized controlled trials (RCTs) have yielded conflicting results. The BEST and BASICS studies failed to show clear superiority of endovascular treatment (EVT) over standard medical care3,4, whereas the BAOCHE and ATTENTION trials demonstrated significant improvement in functional outcomes with MT in carefully selected AVBAO patients with moderate-to-severe stroke (National Institutes of Health Stroke Scale [NIHSS] ≥ 10)5,6.
Despite these advancements, there remains a knowledge gap regarding the outcomes of AVBAO patients with concomitant atrial fibrillation (AF) undergoing EVT. While EVT in anterior circulation strokes has been extensively researched, the posterior circulation, particularly in the context of AF, has received less attention. Major studies on anterior circulation strokes, such as the MR CLEAN trial, HERMES collaboration meta-analysis, and ANGEL-ACT registry, have not shown significant interaction effects between AF and 90-day functional outcomes after EVT7,8. However, these findings may not fully address the unique challenges faced by AVBAO patients with AF due to limitations in sample size, patient heterogeneity, treatment protocols, and the distinct characteristics of posterior circulation strokes.
Literature indicates significantly lower rates of functional independence and higher mortality among AVBAO patients compared to those with anterior circulation strokes9,10,11. However, EVT studies in the posterior circulation face considerable challenges. The paucity of data specifically examining EVT outcomes in AVBAO patients with AF represents a crucial area for investigation, given the potential differences in treatment efficacy across stroke etiologies.
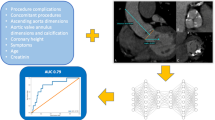
Recent advancements in artificial intelligence (AI) have significantly transformed stroke care, with applications ranging from diagnostic support to personalized treatment strategies. For example, Kim et al. demonstrated the utility of deep learning in LVO detection, while Yao et al. validated the PFCML-MT model for prognosis prediction post-mechanical thrombectomy12,13. Despite these advancements, existing models predominantly address anterior circulation strokes and lack focus on posterior circulation strokes like VBAO with AF. Our study fills this gap by developing a tailored predictive model validated across diverse populations.
To address this critical knowledge gap, our study aims to investigate the impact of EVT on 90-day prognostic outcomes in AVBAO patients with concurrent AF. By focusing on this specific patient cohort, we seek to explore factors potentially influencing post-EVT recovery trajectories. This research aims to contribute to the existing body of knowledge and may provide insights that could inform clinical decision-making, with the goal of supporting optimized therapeutic strategies for AVBAO patients with AF.
Results
Population characteristics
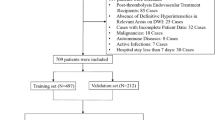
A cohort of 525 patients with VBAO who received EVT were eligible for analysis (Fig. 1), distributed into 368 individuals in the training subset and 157 in the test subset. The baseline characteristics of the study sample are shown in Table 1. In the training subset, for those with favorable outcomes was 62.4 years, and for those with unfavorable outcomes, it was 64.5 years, with the majority being male (69.3% and 70.8%, respectively). Compared to the European cohort, the Chinese cohort had a lower prevalence of atrial fibrillation (22.1% vs 30.5%). The median NIHSS score at admission was recorded as 19 ± 10 for the favorable group and 23 ± 8 for the unfavorable group, while the median posterior circulation Acute Stroke Prognosis Early CT Score (PC-ASPECTS) was noted at 9 ± 1 and 8 ± 2, respectively. In terms of stroke presentation, 48.5% of the favorable group and 52.0% of the unfavorable group exhibited a rapidly progressing stroke. Progressive stroke patterns were observed in 40.4% and 35.1%, fluctuating courses in 4.5% and 4.9%, and wake-up strokes in 6.1% and 8.5% of the respective groups. Of the total patient cohort, 237 patients (18.5%) achieved a favorable outcome, with the rate of symptomatic intracranial hemorrhage (sICH) recorded at 1.8% for the favorable group and 9.9% for the unfavorable group.
This figure outlines the patient selection process for the Chinese and European cohorts, the allocation of training and test sets, and the workflow for model development, validation, and external testing. The Chinese cohort was split into training and test sets, while the European cohort served as an external validation set, highlighting exclusion criteria and key analytical steps.
Table 1 presents the baseline characteristics of patients with unfavorable outcomes (UFO) compared to those with favorable outcomes (FO). The UFO group exhibited more severe initial conditions, including higher NIHSS scores, advanced age, poorer collateral status (American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology [ASITN/SIR] grade 0-1), higher rates of complications like sICH, and more frequent smoking history. Conversely, the FO group demonstrated higher PC-ASPECTS scores, increased rates of intravenous thrombolysis, and shorter estimated occlusion to groin puncture (EOT) times. These findings indicate that the UFO group generally presented with more severe baseline conditions and risk factors, while the FO group benefited from milder initial presentations, more favorable imaging findings, and more timely interventions.
Predictor variable selection
The random forest model identified several predictors significantly associated with the favorable outcome in the dataset. The importance of these predictors was quantified by the increase in Mean Squared Error (MSE) upon permutation, as shown in Supplementary Fig. 1. Variables such as NIHSS, sICH, and EOT were among the top predictors, indicating their substantial influence on the model’s performance. The results revealed that NIHSS, which measures the severity of a stroke, had the highest importance, with an increase in MSE of approximately 12%. This was followed by sICH and EOT, both showing a significant impact on the outcome with increases in MSE around 11% and 9%, respectively. Additional clinical factors, including age, PC-ASPECTS, glucose levels, ASITN/SIR grading, and dyslipidemia, also played roles, albeit with lesser MSE increases (3–8%). The model demonstrated solid performance, with an R² of 0.81, explaining 81% of the outcome variance. Multicollinearity was ruled out, as all Variance Inflation Factor (VIF) values were well below the threshold of 10, ensuring the robustness of the model’s predictive capacity.
Model development and comparison
The initial model (Model 1) included NIHSS, sICH and dyslipidemia as primary predictors, while the extended model (Model 2) added ASITN/SIR, age, glucose, PC-ASPECTS, and the EOT. Upon comparison, Model 2 exhibited superior model fit and predictive efficacy, as evidenced by a lower AIC score (480.3 versus 481.7), a reduced Root Mean Square Error (RMSE: 0.468 versus 0.477), and an elevated Predictive Conditional Probability (PCP: 0.562 versus 0.546) (Fig. 2a). These metrics underscore Model 2’s refined predictive capabilities. Although Model 1 presented a lower Bayesian Information Criterion (BIC), indicative of a more parsimonious model, Model 2’s comprehensive performance metrics and clinical relevance rendered it more appropriate for predictive applications in a clinical context. The improved model provided a more detailed approach to predicting outcomes, and the ggcoef compare function visualized the odds ratios and confidence intervals for each predictor (Fig. 2b), solidifying Model 2 as the final choice detailed in Table 2.
a Comparative Analysis of Model Performance Indices. This figure presents a comparative analysis of two generalized linear models (GLMs), referred to as Model 1 and Model 2, evaluated across diverse performance indices. It aids in identifying the optimal model by striking a balance between predictive accuracy and model complexity. b Model Comparison of Predictor Odds Ratios. The bar chart compares the odds ratios (OR) of predictors in two logistic regression models: a simplified model (Model 1) and a full model (Model 2). ASITN/SIR American Society of Interventional and Therapeutic Neuroradiology score, EOT Time from estimated occlusion to groin puncture, NIHSS National Institutes of Health Stroke Scale score, PC-ASPECTS Posterior circulation Alberta Stroke Program Early CT Score, sICH Symptomatic Intracerebral Hemorrhage.
Model diagnostics
We conducted comprehensive regression diagnostics to assess the reliability of our model (Supplementary Fig. 2). The results supported the model’s robustness, with residuals randomly distributed around zero and most observed data points falling within model-predicted intervals. No substantial collinearity was detected, and influential observations remained within acceptable limits. Furthermore, the consistent alignment of residuals with the quantiles of the standard uniform distribution supported the assumption of homoscedasticity. These diagnostic analyses collectively confirmed that the regression model met key assumptions, thereby validating its reliability and lending credence to the conclusions drawn from it.
Dynamic nomogram
We developed an interactive nomogram using the DynNom function (DynNom package), available at https://ody-wong.shinyapps.io/AF_FO_app/. This tool visualizes the impact of clinical factors on favorable outcomes and aids in predicting stroke prognosis (Supplementary Fig. 3), serving as a clinical decision support component.
Model evaluation
Our logistic regression model, incorporating critical predictive variables, showed strong performance on both a Chinese test dataset and a European external dataset. The model had an AUC of 0.719 with a 95% CI of 0.639–0.799 on the test dataset, and an AUC of 0.684 with a 95% CI of 0.586–0.783 on the external dataset (Fig. 3a, b). DeLong’s test indicated no significant difference in predictive performance between the two datasets (p = 0.512). Our model demonstrated moderate predictive accuracy across two independent datasets, with a C-index of 0.719 for Chinese individuals and 0.684 for Europeans, and a maximum calibration error of 0.088 and 0.091 respectively. The calibration curves (Fig. 3c, d) confirm the model’s alignment with observed outcomes.
a, b ROC Curve Analyses and Optimal Thresholds. a and b display the ROC curve analysis results for the test dataset (a) and the external validation dataset (b), respectively. Calculated using the DeLong method, the AUC for the test dataset is 0.719 (95% CI: 0.639–0.799), with an optimal threshold corresponding to a sensitivity of 0.581 and a specificity of 0.803. For the external validation dataset, the AUC is 0.684 (95% CI: 0.586–0.783), and the optimal threshold point has a sensitivity of 0.562 and a specificity of 0.773. c, d Calibration Curve Analyses. c and d display the calibration curves for the model on the test dataset and the external validation dataset, respectively. The curve for the test dataset deviates from the ideal line to some extent, while the curve for the external validation dataset more closely follows the ideal line, particularly in the higher probability range, indicating better calibration performance for this dataset. e, f Model Diagnostic Performance via DCA Analysis. The DCA analysis diagrams evaluate the diagnostic efficacy of a logistic regression model on a test dataset and an external dataset. The model’s predictions are deemed diagnostically relevant in 82% of cases for the test dataset and 74% for the external dataset.
Decision curve analysis
Decision curve analysis (DCA) on our logistic regression model revealed a 4% optimal net benefit at an 82% threshold in the test dataset, underscoring the model’s diagnostic efficacy. The analysis also indicated a 74% applicability in the external dataset, suggesting sensitivity to threshold probabilities and the model’s generalizability. These results highlight the importance of selecting appropriate nomogram score thresholds for clinical utility across varied settings (Fig. 3e, f).
sICH’s impact on NIHSS prognostic utility
Our comparative analyses showed that in VBAO patients without sICH, higher mRS scores at 3 months were significantly associated with higher NIHSS scores on admission, validating the NIHSS’s prognostic utility (Supplementary Fig. 4). However, in patients with sICH, this correlation was absent, indicating that sICH may reduce the predictive accuracy of the NIHSS for 3-month outcomes.
Model robustness evaluation
To ensure the robustness of our model, we utilized the SampleSizeMLR function, verifying an adequate sample size and statistical power, essential for avoiding overfitting and underpowered analysis. The expR function indicated a very low expected false-positive rate of 0.0082, suggesting a minimal chance of spurious correlations among predictive variables. These rigorous regression analyses confirm the reliability of our model in predicting outcomes for VBAO patients with AF, with an exceptionally low risk of type I errors, reinforcing the model’s validity.
Discussion
This study sought to determine the prognosis of patients with acute VBAO complicated by AF following EVT. Key findings indicate that elevated NIHSS scores and sICH are significantly correlated with adverse 3-month outcomes. Strikingly, a favorable prognosis, defined by a modified Rankin Scale (mRS) score of 0–3, was observed in 47.7% of patients without sICH at the three-month mark. This emphasizes the imperative for early intervention and strategies to reduce sICH risk, which was identified in 7.0% of our patients (aligning with rates from prior research)14. The presence of sICH emerges as a substantial prognostic factor15,16, exerting a significant influence on patient outcomes. These insights are instrumental for refining assessment and treatment strategies to improve clinical management for this patient population.
The NIHSS score, a standard for gauging stroke severity17, reaffirmed its prognostic significance in our study. As per established findings18,19, an elevated NIHSS score correlates with a poorer mRS outcome at three months, underscoring the importance of vigilant post-EVT monitoring and management. Interventions may include stringent blood pressure control, appropriate reversal of anticoagulation in AF patients, and the use of agents like idarucizumab or andexanet alfa for those on direct oral anticoagulants. These targeted strategies may mitigate the adverse effects of sICH, potentially improving functional outcomes and quality of life. Therefore, this underscores the importance of timely and appropriate therapeutic interventions for patients with severe strokes20. However, our findings suggest that in the presence of sICH, the predictive reliability of the NIHSS score for three-month outcomes may be reduced. This raises concerns about the sufficiency of NIHSS in assessing patients with severe complications such as intracranial hemorrhage, indicating a need for additional assessment tools. Our observations imply that the dynamic nature of sICH-related injury may not be fully captured by the initial NIHSS assessment, highlighting a critical area for further research. Future studies should focus on developing modified NIHSS scales or supplementary tools to more accurately reflect the evolving clinical picture in patients with sICH. Moreover, incorporating additional clinical and radiological factors could enhance prognostic accuracy, offering a more comprehensive approach to managing patients with VBAO and AF post-EVT, especially those who develop sICH.
sICH is acknowledged as a pivotal risk factor significantly impacting stroke prognosis21,22,23. Our study’s findings emphasize that among patients with VBAO and AF, sICH is particularly associated with unfavorable functional outcomes. This underscores an immediate necessity for the development of targeted strategies aimed at sICH prevention and management within this specific patient demographic. While recent advancements in anticoagulant therapies have shown promise in reducing sICH risk24,25,26, their effectiveness in the context of posterior circulation strokes, such as VBAO, warrants further investigation.
Our multicenter study, encompassing data from 65 hospitals across China, provides a large and diverse sample, enhancing the generalizability and reliability of our findings. To our knowledge, this is the most extensive cohort study to date on patients with VBAO and AF who have undergone EVT. The external validation using a European cohort further reinforces the generalizability of our results, highlighting their clinical relevance across different populations. Despite the variations in AF incidence between Chinese and European populations27, our predictive model for three-month outcomes post-EVT demonstrated consistent adaptability, underscoring its potential clinical utility across diverse settings.
Furthermore, our methodological rigor adds to the credibility of these findings. The stepwise regression analysis, coupled with thorough sample size verification and beta-error assessment, confirmed the reliability of the selected variables in predicting functional outcomes for patients with VBAO and AF. Importantly, the analysis yielded an exceptionally low expected false-positive correlation rate of 0.0082, minimizing the risk of spurious correlations and further substantiating the robustness of our model for clinical use.
While our analysis identified several factors that showed trends potentially influencing prognosis—such as age, EOT, glucose level, dyslipidemia, and ASITN/SIR grading—none of these reached statistical significance. These findings underscore the need for future research to refine our understanding of prognostic indicators in VBAO with AF patients undergoing EVT. Our study also highlights potential avenues for further investigation, such as imaging biomarkers like CT perfusion PC-ASPECTS and institutional protocols, including the implementation of emergency fast-track systems, which may impact outcomes. Future research should focus on refining these predictors and exploring additional factors that may influence outcomes in this critical patient population.
Our study developed a model highlighting the potential significance of NIHSS scores and sICH in predicting outcomes for VBAO with AF patients undergoing EVT. Our model provides a unique contribution by addressing VBAO complicated by AF, a distinct and underrepresented subgroup in stroke research. By integrating clinical, imaging, and procedural metrics, it enables nuanced risk stratification through capturing complex, nonlinear interactions. Furthermore, our model underwent rigorous external validation using a European cohort, ensuring generalizability across diverse populations. While models like PFCML-MT and MR PREDICTS, among others, have demonstrated significant advancements in predicting outcomes for anterior circulation strokes12,28, our approach focuses on posterior circulation stroke management, complementing existing models by addressing an important clinical gap. This advancement augments the current understanding and offers actionable insights for clinical decision-making. Looking ahead, there is an anticipated benefit from large-scale prospective studies, the refinement of assessment instruments, and the customization of treatment strategies. These enhancements are poised to further optimize the prognosis for individuals within this patient cohort. The consistency observed across datasets suggests that the model demonstrates promising predictive capabilities across diverse ethnic populations, reinforcing the potential for its application in a global context. This cross-ethnic applicability potentially warrants further investigation for broader clinical prognostic applications.
While our study presents valuable findings, it is not without limitations. Retrospective in nature, it is susceptible to inherent selection bias and potential confounding factors that could not be fully accounted for. Moreover, the utilization of an external validation set, though beneficial for generalizing results, was constrained by a limited sample size. Additionally, this study focused only on the NIHSS score and sICH, without comprehensively exploring other potential influencing factors. Future research should aim for multicenter, large-scale prospective studies to delve deeper into the complexities of VBAO and AF in the context of EVT. Future iterations of our predictive model will incorporate advanced imaging biomarkers, including CT perfusion metrics, MRI-based perfusion imaging, and radiomics features, to enhance its prognostic accuracy. Additionally, we are actively expanding our dataset through multicenter collaborations to include larger and more diverse patient cohorts. These efforts aim to further validate the model prospectively and ensure its applicability across global clinical settings. Prospective validation is critical to confirm the robustness and clinical utility of our predictive model. We have initiated a large-scale, multicenter, prospective cohort study (Registration Number: ChiCTR2300074824; and registration website: www.chictr.org.cn) to comprehensively evaluate its performance in real-world clinical settings. Furthermore, embedding this model into clinical trials and seamlessly integrating it into routine clinical workflows may further establish its role in guiding personalized therapeutic strategies for VBAO patients with AF. Another methodological limitation involves balancing predictive performance with model interpretability when selecting machine learning approaches. While Random Forests demonstrates exceptional capability in capturing complex variable interactions across diverse data types, they inherently present challenges in direct interpretability compared to traditional logistic regression models. To address this analytical complexity, we implemented a strategic two-stage modeling approach: employing Random Forests for comprehensive feature exploration, followed by integrating statistically significant predictors into a logistic regression framework, thus synthesizing advanced predictive techniques with established statistical transparency.
This study lays a foundational framework for improving the evaluation and management of patients with VBAO and AF following EVT. For such a model to be feasible for future implementation, integration into electronic health record systems will be required to automate the data collection process necessary for recurrent predictions. Future efforts should focus on enhancing scoring systems, optimizing anticoagulation protocols, and personalizing therapeutic approaches. Additionally, streamlining the integration of machine learning models into electronic health records is likely to become a key area of research and development. These advancements collectively hold the potential to significantly improve survival rates and quality of life for this unique patient population while supporting the effective deployment of predictive models in clinical practice.
Methods
Study design and participants
This investigation adopted a two-stage, multicenter, retrospective approach, encompassing 65 centers across 15 provinces in China, and spanned from December 2015 to June 2022, utilizing data from the PostErior ciRculation iSchemIc Stroke regisTry (PERSIST) (Registered at URL: http://www.chictr. org.cn/; Unique identifier: ChiCTR2000033211). Comprehensive details about the participant enrollment procedure are documented in prior publications29,30. Eligibility for inclusion in the study was determined by the following criteria for patients with VBAO who received EVT: (1) age ≥18 years; (2) presence of concurrent AF; (3) received EVT; (4) VBAO confirmed by CT angiography, MR angiography, or digital subtraction angiography (DSA); and (5) presentation within 24 h post-estimated VBAO onset. Exclusion criteria were: (1) a pre-stroke modified Rankin Scale (mRS) score >2; (2) concurrent anterior circulation stroke, arteriovenous malformation, or aneurysm; (3) participation in any clinical trial; (4) pregnancy or lactation; and (5) incomplete baseline critical data and imaging. Additionally, to ensure the external validity of our findings, we incorporated an external validation subset comprising 237 individuals from a distinct European population.
Ethical considerations
This study was conducted with human participants and received approval from the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (USTC) in Hefei, China (No. 2020ky056). Following national legislation and institutional protocols, the requirement for written informed consent was waived for this research.
Data collection
Demographic data, including vascular risk factors, laboratory results, neuroimaging findings, temporal metrics such as time from estimated occlusion to groin puncture (EOT), and stroke severity, were meticulously compiled from a retrospective review of medical records. Arterial occlusion sites were systematically classified into the following segments: Basilar Artery Remote (distal segment of the basilar artery, BA Remote), Basilar Artery Mid (mid-segment of the basilar artery, BA Mid), Basilar Artery Proximal (proximal segment of the basilar artery, BA Proximal), and Vertebral Artery segment 4 (the fourth segment of the vertebral artery, V4). This classification scheme facilitated the accurate identification and localization of arterial obstructions. The severity of stroke was evaluated using the NIHSS score. Collateral circulation status was evaluated following the ASITN/SIR criteria. PC-ASPECTS was determined using non-contrast CT or MRI. sICH was diagnosed utilizing the Heidelberg Bleeding Classification, which was applied when new imaging evidence of hemorrhage corresponded with a notable escalation in the NIHSS score or a deterioration triggered by interventions such as hemicraniectomy, intubation, or placement of an external ventricular drain, in the absence of other attributable causes for the clinical decline31.
Radiologic evaluation
The imaging core laboratory conducted evaluations on baseline noncontrast computed tomography (NCCT) or diffusion-weighted magnetic resonance imaging (DWI) to assess PC-ASPECTS, baseline vessel imaging (CTA, MRA, or DSA) to identify the occlusion location, and angiographic outcomes on DSA for technical efficacy regarding reperfusion. Follow-up CTA or MRA within 48 h was used to assess vessel recanalization, and follow-up CT was examined for the presence of ICH. All neuroimaging assessments were performed independently by two neuroradiologists who were blinded to the treatment group assignments, clinical data, and outcomes. In cases of discordance, a third experienced neuroradiologist adjudicated the final decision.
Endovascular treatment
The therapeutic regimen encompassed a spectrum of endovascular strategies, including mechanical thrombectomy with stent retrievers and/or thromboaspiration, stenting, balloon angioplasty, intra-arterial thrombolysis, or a combination thereof. The selection of a specific endovascular intervention at each participating facility was made by a consortium of neurointerventionists and neurologists, following a thorough evaluation and in accordance with the patients’ wishes or the directives of their legal representatives. The decision-making process for treatment modalities was rooted in a comprehensive assessment of the patient’s overall condition, with the goal of delivering individualized and optimal care.
Functional outcome assessment
The follow-up protocol involved assessing patients’ functional status with the mRS at discharge and at the three-month interval following stroke. A favorable outcome was defined as an mRS score ranging from 0 to 3, indicating functional independence. The mRS evaluations were conducted by certified assessors, who remained blinded to the study’s objectives and performed their assessments either during outpatient visits or through telephone interviews with the patients or their designated caregivers.
Statistical analysis
Statistical analyses were executed utilizing IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY, USA), in conjunction with R statistical software, version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). The normality of the distribution for continuous variables was evaluated employing the one-sample Kolmogorov–Smirnov test. Variables presenting a normal distribution were reported as mean ± standard deviation (SD), while those exhibiting non-normal distribution were detailed as median (interquartile range [IQR]). Missing data were addressed using the ‘missRanger’ package in R, which employs chained random forests for multiple imputation. This method iteratively imputes missing values by modeling each variable with missing data as a function of other variables, effectively capturing complex, non-linear relationships within the data32. A random seed was set (‘set.seed(1234)’) to ensure reproducibility, and out-of-bag error estimates were utilized to assess imputation accuracy. (1) Sample Grouping: Participants were randomly allocated into training and test cohorts in a 7:3 ratio, employing a stratified methodology pivotal for the generation of a dependable dataset amenable to model development and validation endeavors. To ensure model robustness and generalizability, the dataset was stratified by outcome groups (favorable vs. unfavorable) and randomly split into training (70%) and validation (30%) subsets. The training set supported model development and variable selection, while the validation set was used for independent evaluation of metrics such as AUC and calibration. This approach mitigates overfitting, improves generalizability, and ensures unbiased performance assessment. (2) Data Analysis in the Training Set: Random Forest permutation importance analysis was performed using R (rfPermute package) to evaluate variable importance for predicting favorable outcome. The model used 500 trees and 100 permutation repetitions. Variable importance was assessed by percentage increase in Mean Squared Error (%IncMSE) and visualized using ggplot2. Significance levels (***p < 0.001, **p < 0.01, *p < 0.05) were indicated. Variables with p < 0.05 were included in a multivariable logistic regression model. (3) Dataset Validation: In addition to internal validation (test dataset), we expanded our model’s validation across diverse geographical regions beyond Asia. To achieve this, we retrospectively collected data from VBAO patients with concurrent AF who underwent EVT at 16 comprehensive stroke centers across Italy, France, and Poland from December 2015 to December 2023. Our stringent adherence to the inclusion and exclusion criteria followed the established guidelines outlined in PERSIST. This multi-center approach ensured the compilation of a robust and varied dataset crucial for external validation. (4) Model Assessment: The accuracy of the logistic regression model was meticulously assessed through several metrics: the area under the receiver operating characteristic curve (AUC), the calibration curve, and the decision curve analysis (DCA). These metrics offered a comprehensive assessment of the model’s prognostic abilities. Two-sided P values < 0.05 were considered statistically significant.
Data availability
In the interest of promoting scientific transparency and reproducibility, the principal investigators of the PERSIST study are committed to sharing the deidentified individual participant data (IPD). Access to the IPD will be provided upon request for researchers who wish to conduct further analyses, subject to approval by an independent review committee.
Code availability
No code was written by authors.
References
Singer, O. C. et al. Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann. Neurol. 77, 415–424 (2015).
Nguyen, T. N. & Strbian, D. Endovascular Therapy for Stroke due to Basilar Artery Occlusion: A BASIC Challenge at BEST. Stroke 52, 3410–3413 (2021).
Liu, X. et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 19, 115–122 (2020).
Langezaal, L. C. M. et al. Endovascular Therapy for Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 384, 1910–1920 (2021).
Jovin, T. G. et al. Trial of Thrombectomy 6 to 24 h after Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 387, 1373–1384 (2022).
Tao, C. et al. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. N. Engl. J. Med. 387, 1361–1372 (2022).
Smaal, J. A. et al. Effect of atrial fibrillation on endovascular thrombectomy for acute ischemic stroke. A meta-analysis of individual patient data from six randomised trials: Results from the HERMES collaboration. Eur. Stroke J. 5, 245–251 (2020).
Tong, X. et al. Endovascular treatment for acute ischemic stroke in patients with versus without atrial fibrillation: a matched-control study. BMC Neurol. 21, 377 (2021).
Yang, Y. et al. The effects of pharmaceutical thrombolysis and multi-modal therapy on patients with acute posterior circulation ischemic stroke: Results of a one center retrospective study. Int. J. Surg. 39, 197–201 (2017).
Liu, H. et al. Endovascular treatment for acute basilar artery occlusion due to different stroke etiologies of large artery atherosclerosis and cardioembolism. Eur. Stroke J. 7, 238–247 (2022).
Zhang, J., Wang, Y., Ju, Y. & Jiang, H. Endovascular treatment of acute basilar artery occlusion: A systematic review and meta-analysis of first-line stent retriever versus direct aspiration. Brain Behav. 13, e3141 (2023).
Yao, Z., Mao, C., Ke, Z. & Xu, Y. An explainable machine learning model for predicting the outcome of ischemic stroke after mechanical thrombectomy. J. Neurointerv. Surg. 15, 1136–1141 (2023).
Kim, J. G. et al. Automated detection of large vessel occlusion using deep learning: a pivotal multicenter study and reader performance study. J. Neurointerv. Surg., https://doi.org/10.1136/jnis-2024-022254 (2024).
Sun, W. et al. The safety and effectiveness of endovascular treatment for patients with vertebrobasilar artery occlusions: according to the BEST and BASICS criteria. Ther. Adv. Neurol. Disord. 15, 17562864221114627 (2022).
Huang, P. & Yi, X. Y. Predictive role of admission serum glucose, baseline NIHSS score, and fibrinogen on hemorrhagic transformation after intravenous thrombolysis with alteplase in acute ischemic stroke. Eur. Rev. Med. Pharmacol. Sci. 27, 9710–9720 (2023).
Yang, Y. et al. Blood glucose to predict symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke with large infarct core: a prospective observational study. Front. Neurol. 15, 1367177 (2024).
Bellomo, J. et al. The volume of steal phenomenon is associated with neurological deterioration in patients with large-vessel occlusion minor stroke not eligible for thrombectomy. Eur. Stroke J. https://doi.org/10.1177/23969873241251718 (2024).
Farooqui, M. et al. Anesthetic management for large vessel occlusion acute ischemic stroke with tandem lesions. J. Neurointerv. Surg. https://doi.org/10.1136/jnis-2023-021360 (2024).
Sanak, D. et al. Quality of Life in Patients with Excellent 3-Month Clinical Outcome after First-Ever Ischemic Stroke: A Time to Redefine Excellent Outcome? Eur. Neurol. 87, 1–10 (2024).
Levi, A. et al. Management and Outcome of Acute Ischemic Stroke Complicating Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 15, 1808–1819 (2022).
Mako, M. et al. Predictors of Outcome after Direct Aspiration of Basilar Artery Occlusion. J. Clin. Med. 13, 1576 (2024).
Ni, H. et al. Technical Risk Stratification Nomogram Model for 90-Day Mortality Prediction in Patients With Acute Basilar Artery Occlusion Undergoing Endovascular Thrombectomy: A Multicenter Cohort Study. J. Am. Heart Assoc. 13, e032107 (2024).
Gallego-Fabrega, C. et al. A multitrait genetic study of hemostatic factors and hemorrhagic transformation after stroke treatment. J. Thromb. Haemost. 22, 936–950 (2024).
Bhatia, K. et al. Contemporary Antiplatelet and Anticoagulant Therapies for Secondary Stroke Prevention: A Narrative Review of Current Literature and Guidelines. Curr. Neurol. Neurosci. Rep. 23, 235–262 (2023).
Fischer, U. et al. Early versus Later Anticoagulation for Stroke with Atrial Fibrillation. N. Engl. J. Med. 388, 2411–2421 (2023).
Mac Grory, B. et al. Recent Vitamin K Antagonist Use and Intracranial Hemorrhage After Endovascular Thrombectomy for Acute Ischemic Stroke. JAMA 329, 2038–2049 (2023).
Vervoort, D. et al. Tertiary prevention and treatment of rheumatic heart disease: a National Heart, Lung, and Blood Institute working group summary. BMJ Glob Health 8, e012355 (2023).
Kremers, F. et al. Outcome Prediction Models for Endovascular Treatment of Ischemic Stroke: Systematic Review and External Validation. Stroke 53, 825–836 (2022).
Huang, Z. X. et al. Prognostic factors for acute vertebrobasilar artery occlusion-reperfusion: a multicenter retrospective cohort study. Int. J. Surg. 109, 2303–2311 (2023).
Xu, Y. et al. Effect of INR on Outcomes of Endovascular Treatment for Acute Vertebrobasilar Artery Occlusion. Transl. Stroke Res. https://doi.org/10.1007/s12975-023-01176-y (2023).
Xu, Y. et al. Temporal progression of functional independence after mechanical thrombectomy in acute vertebrobasilar artery occlusions. J. Neurointerv Surg. https://doi.org/10.1136/jnis-2023-020939 (2023).
Stekhoven, D. J. & Buhlmann, P. MissForest-non-parametric missing value imputation for mixed-type data. Bioinformatics 28, 112–118 (2012).
Acknowledgements
The study was supported by Research Funds of Centre for Leading Medicine and Advanced Technologies of IHM (No. 2023IHM01050 and 2023IHM01052), and the Science and Technology Program of Guangzhou, China (2024B03J0436).
Author information
Authors and Affiliations
Contributions
Z.X.H., A.M.A., A.P. designed the study and initial analysis plan. Z.X.H., A.M.A., A.P. contributed to the statistical analysis, performed the data analysis, and wrote the draft of the manuscript. X.H., W.S. contributed to revision of the manuscript. All authors mentioned above (Z.X.H., A.M.A., A.P., X.H., Q.H., Y.L., P.C., A.B., L.S., S.A., C.C., M.B., A.R., E.K., G.F., G.M., S.S., M.P.G., L.B., B.D.S., F.A., Susanna S., A.S., S.L.V., G.C., L.R., X.H., W.S.) made substantial contributions to the content of the paper, including the acquisition of data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, ZX., Alexandre, A.M., Pedicelli, A. et al. AI prediction model for endovascular treatment of vertebrobasilar occlusion with atrial fibrillation. npj Digit. Med. 8, 78 (2025). https://doi.org/10.1038/s41746-025-01478-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-025-01478-5