Abstract
Despite much promise in overcoming drug-resistant infections, clinical studies of bacteriophage antibacterial therapy have failed to show durable effectiveness. Although lysogeny plays an important role in bacterial physiology, its significance in diverse microbiomes remains unclear. Here, we tested the following hypotheses: 1) urinary microbiome phage populations switch to a higher relative proportion of temperate phages, and 2) the activity of the phage recombination machinery (integration/excision/transposition) is higher during human urinary tract infections (UTIs) than in non-infected urinary tracts. Using human urine, model organisms, mass spectrometry, gene expression analysis, and the phage phenotype prediction model BACPHLIP, the results corroborated our hypotheses at the functional protein and gene levels. From a human health perspective, these data suggest that temperate phages may facilitate bacterial infections rather than function as protective agents. These findings support the use of lysogenic phages as therapeutic Trojan Horses.
Similar content being viewed by others
Introduction
The resistance of bacteria to antibiotics exerts a heavy economic and human health burden on society; therefore, it is imperative to explore new ways to target multi-drug-resistant bacteria. Bacteria are the natural prey of bacteriophages. Consequently, bacteriophage therapy has long been pursued as a treatment modality for antibiotic-resistant bacterial infections, including urinary tract infections (UTIs)1. Despite its promise, clinical experimental phage anti-bacterial UTI therapy has failed to show a significant durable effectiveness1,2. Could lysogeny contribute to pathogen resistance mechanisms in vivo? Currently, functional phenotypic data collected during human UTI bacterial infections is insufficient to answer this question.
In this study, we focused on two hypotheses: 1) urinary microbiome phage populations switch to a higher relative proportion of temperate phages and 2) the activity of the phage recombination machinery (integration/excision/transposition) is higher during human urinary tract infections (UTIs) than in non-infected individuals. These hypotheses underly the previous speculations that lysogeny plays a role during human infection3 and are inspired by previous work in marine ecosystems4,5. Marine scientists proposed a model where high-density microbial communities switch to lysogeny4 and highlighted the need of rigorous, system-level data to support model generalization to non-coral reef environments5.
Bacteriophages can exhibit a lytic or lysogenic life cycle6,7. Lytic bacteriophages invade their host and replicate during the lytic cycle, a process that involves producing new viral offspring and releasing them from the infected host6. Temperate phages are those whose DNA is integrated into the genome of the host prey after invasion and can be replicated synchronously with the host bacterial DNA (lysogeny)8. Under host stress or competition for limited resources, temperate phages can exit the lysogenic state and produce more lytic virions6. The enrichment of lysogens can also herald a cooperation stage between the phage and its prey, thereby accelerating the human infection9. While this theory is attractive, data collected during the onset of human bacterial infections has been very limited.
A functional characterization of the influence of UTIs on the urinary tract phage microbiome has not been completed in prior research studies because of overarching technical challenges6. To overcome the analytical challenges, we developed a culture-free mass spectrometry workflow that ensures high analytical sensitivity, specificity, and rapid throughput. The approach has been successfully validated by the authors of this manuscript in prior clinical studies involving patients with tuberculosis, Lyme disease, and toxoplasmosis10,11,12,13.
Past phage research used genomic data to study lysogeny3. In contrast, in this study, we added the additional dimension of functional proteomics to provide a rigorous test of our hypotheses. We employed affinity capture-enriched liquid chromatography tandem mass spectrometry (LC-MS/MS) to perform proteomic analysis of the urine of UTI patients and controls. The phage and uropathogenic bacteria protein abundances, and the phage lifestyle predicted in silico using the Bacphlip algorithm14, were correlated to the presence or absence of UTI. Phages were isolated from human urine by plaque formation assays and electron microscopy. Finally, we used uropathogenic Escherichia coli (UPEC) CFT073 [WAM2267] and E. coli B organisms to monitor the prophage recombination activity (integration, excision, and transposition) in model solutions containing UTI and non-UTI control urine.
Results
Protein affinity enrichment affords high analytical sensitivity of mass spectrometry analysis
In order to identify low-abundance proteins derived from phages and bacteria in presence of a vast excess of human proteins in the urine matrix, it is necessary to increase the dynamic range afforded by tandem mass spectrometry proteomic analysis. Affinity capture has been shown to successfully address this issue and to effectively increase the analytical sensitivity of protein detection15. In this study, poly(NIPAm/AA/RB221) affinity hydrogel particles were used to achieve a 10 fold increase of protein detection analytical sensitivity (Supplementary Fig. 1). Experiments using T3 phage protein lysate spiked in 1 mL of urine at a concentration of 2 and 0.2 ng/µL, showed that the affinity particles coupled to discovery mass spectrometry analysis yielded a total of 20 phage proteins and identified 6 proteins at the phage protein lowest concentration (Supplementary Data 1). These results support the capability of the method to identify low-abundance phage proteins in a complex urine matrix containing vast excess of human proteins.
Clinical urine samples (40 mL) were enriched with poly(NIPAm/AA/RB221) affinity particles and subjected to discovery mass spectrometry analysis using NCBI and Uniprot Homo sapiens, uropathogenic Escherichia coli (UPEC), Escherichia coli 0157, Escherichia coli O25b, Enterobacter cloacae, Enterococcus faecalis, Staphylococcus aureus, Streptococcus agalactiae, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Klebsiella pneumoniae IS43UP, and Bacteriophages databases (Fig. 1). The affinity-enriched mass spectrometry analysis identified 143, 845, and 3216 protein groups for bacteriophages, urinary tract infection pathogens, and Homo sapiens, respectively. (Supplementary Fig. 1B, Supplementary Data 2–4).
Affinity hydrogel spheres are mixed with urine samples; captured proteins are eluted and processed for discovery mass spectrometry proteomic analysis. Tandem spectra are matched with a protein database of bacteriophages, Homo sapiens, and of UTI pathogens (uropathogenic Escherichia coli UPEC, Escherichia coli 0157, Escherichia coli O25b, Enterobacter cloacae, Enterococcus faecalis, Staphylococcus aureus, Streptococcus agalactiae, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Bacteriophages databases) retrieved from the Uniprot and NCBI repositories. Created in https://BioRender.com.
Phage protein abundance linearly correlates with bacterial protein abundance, but the relative amount of phage per bacteria decreases as bacterial abundance increases
We identified 143 and 845 protein groups attributed to bacteriophages and uropathogenic organisms, respectively. In order to characterize the relationship between phage and pathogen protein abundance, we performed a Pearson correlation analysis. Overall, considering all uropathogenic bacteria and their phages, the phage viroproteome abundance correlated with the UTI pathogen protein abundance (Pearson r = 0.43, p = 0.02, Fig. 2A). The correlation was maintained when analyzing the protein abundance of UPEC and its phages (Pearson r = 0.87, p = 5e-10, Fig. 2B). Figure 2 shows the plot of phage protein abundance versus bacterial protein abundance, where UTI patients are depicted in blue, in-country controls are depicted in red, and US controls are depicted in green. As expected due to a higher bacterial load, UTI patients tend to have higher pathogen and phage protein abundance than non-UTI controls (Fig. 2A,B). The slope of a linear regression line for all uropathogens and UPEC was less than 1 (m = 0.65 and 0.88; p = 0.0026, 1e-9, respectively, Fig. 2A,B). This reflects a relative decline of lytic viruses with increasing bacterial host density and supports our hypothesis of a diminished proportion of lytic phages and a higher proportion of lysogenic phages at high bacterial densities.
Phages that are annotated to infect uropathogenic bacterial are considered here. A, B The protein abundance of bacteria versus phages in UTI cases and controls are plotted; every dot is a patient. Grey lines depict 1:1 linear relationship. Slope m and R2 values refer to linear regressions; P values are derived from a two-sided t-test against a slope ≠ 1. Protein abundance of all uropathogenic bacteria considered (uropathogenic Escherichia coli (UPEC), Enterobacter cloacae, Enterococcus faecalis, Staphylococcus aureus, Streptococcus agalactiae, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae) and their phages is reported in (A), protein abundance of E. coli and its phages is reported in (B). C UTI patients had a higher likelihood of temperate phenotype phage protein detection compared to non-UTI controls (odd ratio = 1.47, 95% CI 1.11–2.11, Fisher exact test p = 0.03). The phage phenotype was predicted by BACPHLIP14.
A LASSO logistic regression model was used to identify a reduced set of combined phage and pathogen proteins that could discriminate patients based on their UTI status. A set of 14 proteins (Supplementary Results 1, Supplementary Table 1, Supplementary Fig. 2) was identified that could correctly discriminate between cases and controls with 100% accuracy: Escherichia coli Colicin I receptor P17315, Escherichia coli Cysteine desulfurase A0A376Y6R3, Escherichia coli Large ribosomal subunit protein uL24 A0A2X1JEL2, Xanthomonas phage Xoo-sp14 Uncharacterized protein A0A7H0XAJ2, Pseudomonas aeruginosa YfdX protein A0A241XSA9, Bacteriophage sp MAG TPA: endosialidase chaperone, DAQ14039.1, Escherichia coli Putative pesticin receptor A0A0H2V8C1, Vibrio phage H188 Membrane lipoprotein A0A0K1LKF6, Lactococcus phage N4-gp56 family major capsid protein, Q9AZ61, Proteus phage Myduc uncharacterized protein CPT_Myduc_012, QFG06635.1, Escherichia coli Large ribosomal subunit protein uL2 A0A377K133, Enterococcus phage vB_EfaS_AL2 hypothetical protein FDJ52_gp08, YP_009624796.1, Enterococcus faecalis cyclic pyranopterin monophosphate synthase MoaC WP_172503165, Klebsiella sp. Cytochrome b561 homolog 2 A0A7H4N9H1.
UTI patients have a higher likelihood to harbor phages with a temperate lifestyle
Identified phage proteins belong to 114 phage species (Supplementary Data 2). We used the published BACPHLIP algorithm14 to predict the phenotype of phages identified in the urine of UTI patients and controls (Supplementary Data 5). BACHLIP analyzes conserved protein domains to achieve a phage lifestyle prediction accuracy of 98%14. According to BACPHLIP predictions, the ratio of lysogenic to lytic phages was 28% or 25/89. When analyzing the distribution of phage proteins in UTI versus non-UTI patients according to the phage-predicted lifestyle, we see that UTI patients have a higher relative likelihood of harboring phages with a temperate lifestyle compared to non-UTI controls (odd ratio = 1.47, 95% CI 1.11–2.11, Fisher exact test p = 0.03, Fig. 2C).
The bacteriophage urinary viroproteome is differentially modulated in UTI versus non-UTI patients
The urine sample analysis identified 143 protein groups that were attributed to bacteriophages (Fig. 3A, Supplementary Fig. 3). A Venn diagram revealed that 3 proteins were unique to UTI patients, 1 protein was unique to non-UTI controls in the CoG, and 14 proteins were unique to non-UTI controls from the United States, while 66% of identified phage proteins were shared in the three classes (Fig. 3B). Phage protein abundance trended higher in UTI patients than in non-UTI controls (ANOVA F = 5.899, p = 0.003, post hoc pairwise t-test with Holm’s correction p = 0.002 and 0.09 for UTI versus nonUTI.CoG and nonUTI.US, respectively, Supplementary Data 2). The total levels of phage proteins trended higher in UTI cases than controls, although this difference was not statistically significant (ANOVA F = 2.292, p = 0.12, post hoc pairwise t-test with Holm’s correction p = 0.13 and 0.44 for UTI versus nonUTI.CoG and nonUTI.US, respectively, Fig. 3B). A three-way limma analysis16 identified differentially abundant proteins (Fig. 3C) in UTI (blue) versus in-country controls (red) and controls collected in the US (green, Fig. 3D, Supplementary Data 4). The number of phage proteins more abundant in UTI than CoG non-UTI patients was 10 (limma p-value < 0.05, Supplementary Data 6), while 16 proteins were more abundant in CoG non-UTI controls (limma p-value < 0.05, Supplementary Data 4). The number of phage proteins more abundant in UTI than US non-UTI patients was 23 (limma p-value < 0.05), and the number of proteins more abundant in US non-UTI controls was 37 (limma p-value < 0.05, Supplementary Table 2, Supplementary Data 6). The limma analysis highlights how there is a larger number of overexpressed phage proteins in non-UTI samples than in UTI samples, thus supporting the hypothesis of a larger lytic presence in the phage microbiome of individuals void of UTI. The biological processes of the identified proteins were diverse and included metabolism processes of DNA and RNA, cell organization and biogenesis, signal transduction, and others (Fig. 3E-F). Identified proteins included several types of structural proteins such as phage head morphogenesis proteins, bond cleaving proteins such as peptidoglycan hydrolase responsible for peptidoglycan chains bond cleavage2 and cytolethal descending factor (cdf) toxin V (Supplementary Fig. 4). A LASSO logistic regression identified a reduced set of phage proteins that could discriminate patients based on their UTI status (Supplementary Results 2, Supplementary Table 2, Supplementary Fig. 5).
A Venn diagram shows that 66% of identified phage proteins are shared by the three patient classes (UTI, in-country non-UTI controls, US non-UTI controls). 3, 1, and 14 proteins are only found in UTI patients, in-country non-UTI controls, US non-UTI controls, respectively. B Total levels of phage protein abundance in UTI, non-UTI, CoG and non-UTI, US patients. One point corresponds to one patient. ANOVA F = 2.292, p = 0.12, post hoc pairwise t-test with Holm’s correction p = 0.13 and 0.44 for UTI versus nonUTI.CoG and nonUTI.US, respectively. C Identified phage proteins are involved in DNA and RNA metabolism, virion assembly and attachment. D–F Three-way limma analysis highlights differentially abundant proteins in UTI (blue) versus in country controls (red) and controls collected in the US (green). G Pictorial representation of identified phage proteins contextualized in the predicted cellular compartments. Created in https://BioRender.com.
Functionally important proteins related to each bacteriophage lifecycle phase were identified in the urine of UTI patients and controls (Supplementary Results 3). If confirmed, these proteins can help elucidate the dynamics of phage populations and their impact on microbial communities, can serve to characterize and monitor molecular mechanisms lifecycle phases in vivo and gain insights into phage-host interactions. Proteins mediating phage adsorption, penetration, replication, recombination, repair, maturation, and release6 were identified. Moreover, proteins involved in lysogenic life cycle mechanisms, including phage integration, recombination, and lysogenic conversion were identified (Supplementary Results 3).
The UTI pathogen proteome is differentially modulated in the urine of UTI patients versus non-UTI controls
The urine sample analysis identified 845 protein groups that were attributed to UTI-causing pathogens (UPEC, Klebsiella pneumonia, Pseudomonas aeruginosa, Enterococcus faecalis, Proteus mirabilis, Supplementary Fig. 6, Supplementary Data 3). A Venn diagram revealed that 67 proteins were unique to UTI patients, 3 proteins were unique to non-UTI controls in the CoG, and 10 proteins were unique to non-UTI controls from the United States, while 66% of identified pathogen proteins were shared in the three classes (Fig. 4A). Bacteria protein abundance trended higher in UTI patients than in non-UTI controls (ANOVA F = 7.987, p = 0.0003, post hoc pairwise t-test with Holm’s correction p = 0.07 and 0.0002 for UTI versus nonUTI.CoG and nonUTI.US, respectively, Supplementary Data 3). The total levels of bacterial proteins trended higher in UTI cases than controls (ANOVA F = 9.669, p = 0.000681, post hoc pairwise t-test with Holm’s correction p = 0.06 and 0.0005 for UTI versus nonUTI.CoG and nonUTI.US, respectively, Fig. 4B). Proteins from UTI pathogens were identified in the urine samples with different frequencies, where UPEC is the most frequent and Proteus mirabilis is the less frequent (Fig. 4C). A three-way limma analysis16 identified differentially abundant proteins in UTI (blue) versus in-country controls (red) and controls collected in the US (green, Fig. 4D–F, Supplementary Data 7). The number of bacterial proteins more abundant in UTI patients than in CoG controls was 62 (limma p-value < 0.05, Supplementary Data 7), while CoG non-UTI controls than in UTI patients was 10 (limma p-value < 0.05, Supplementary Data 7). The number of phage proteins more abundant in UTI than in US non-UTI patients was 174 (limma p-value < 0.05, Supplementary Data 7), and the number of proteins more abundant in US non-UTI controls was 17 (limma p-value < 0.05, Supplementary Data 7). The limma analysis highlights how there is a larger number of overexpressed bacterial proteins in UTI samples than in non-UTI samples, thus supporting a larger bacterial presence in patients with UTI. Identified proteins were involved in metabolic processes, transport, translation, and transcriptional regulation (Fig. 4G). A LASSO logistic regression identified a reduced set of bacterial proteins that could discriminate patients based on their UTI status (Supplementary Results 4, Supplementary Table 3, Supplementary Fig. 7).
A Venn diagram shows that 66% of identified pathogen proteins are shared by the three patient classes (UTI, in-country non-UTI controls, US non-UTI controls). 67, 3, and 10 proteins are only found in UTI patients, in-country non-UTI controls, US non-UTI controls, respectively. B Total levels of bacterial protein abundance in UTI, non-UTI, CoG and non-UTI, US patients. One point corresponds to one patient. ANOVA F = 9.669, p = 0.000681, post hoc pairwise t-test with Holm’s correction p = 0.06 and 0.0005 for UTI versus nonUTI.CoG and nonUTI.US, respectively. C Proteins from UPEC, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecalis, and Proteus mirabilis were identified in the urine samples with different frequencies. D–F Three-way limma analysis highlights differentially abundant proteins in UTI (blue) versus in-country controls (red) and controls collected in the US (green). G Proteins are involved in metabolic processes, transport, translation, and DNA/RNA metabolism.
Functionally important proteins related to all known mechanisms of bacterial resistance to phage infection were identified in the urine of UTI patients and controls, revealing the dynamic ecology of the infection (Supplementary Results 5). Proteins mediating the following anti-phage defenses were identified: a) bacterial cell surface alterations to avoid phage adsorption and prevention of phage DNA injection, b) restriction of incoming DNA, c) blocking phage assembly, d) acquiring phage-specific immunity through clustered regularly interspaced short palindromic repeats and abortive infection17 (Abi, Supplementary Results 5).
Phages can be directly isolated from healthy and UTI urine using an agar overlay assay
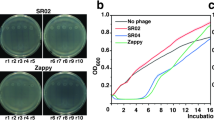
We isolated phages from two patients (patient IDs F31 and F8 in Table 1) using the classic agar overlay method (Fig. 5A). Using the agar overlay isolation, two morphotypes of phages were identified, Siphoviridae and Podoviridae (Fig. 5B, C). Siphoviridae phages were isolated from a healthy 10-year-old female (Fig. 5B, patient ID F31). The plaques from Siphoviridae phages had a 1 mm diameter. Podoviridae phages were isolated from an adult male patient with UTI (Fig. 5C, patient ID F8). Phages with such a phenotype are rarely observed in environmental isolates. The plaques of Podoviridae phages were almost all identical with a turbid center and a 1–4 mm diameter. The titer of these phages was 1010 pfu/mL on day one and was unstable and dropped 3 logs within several days. Electron microscopy imaging was performed on the clinical isolates and confirmed that the clinical isolates contained intact phage particles (Fig. 5C, D). Phage isolation from urine can be enhanced using affinity hydrogel particle precipitation, which achieved 82% phage recovery efficiency from a complex biological matrix as demonstrated using soft agar plating and plaque counting (Supplementary Results 6, Supplementary Fig. 8).
A Phages were isolated from urine using a standard overlay technique and characterized using transmission electron microscopy (TEM), created in https://BioRender.com. B, C Plaque assays of phage isolated from human urine, digital camera images. Scale bar = 3 mm. D, E TEM images of phage clinical isolates. Scale bar = 100 nm and 50 nm, in (D and E), respectively.
E. coli spiked in UTI urine has more prophage integration, excision, and transposition activity than E. coli spiked in non-UTI urine
In order to discern the effect that UTI urine has on temperate phages, E. coli B and UPEC E. coli CFT073 [WAM2267] were allowed to grow in presence of urine collected from UTI (OD600 = 0.28–0.31) and non-UTI (OD600 = 0) individuals (Fig. 6A). Bacteria were spiked at a concentration of 108 CFU/mL in a total volume of 5 mL per incubation. The OD600 readings of the bacterial suspensions were as follows: CFT073 spiked in normal urine (N-CFT073) = 0.59, E. coli B spiked in normal urine (N-E.coli) = 0.72–0.8, CFT073 spiked in UTI urine (U-CTF073) = 0.76–0.8, E. coli B spiked in UTI urine (U-E.coli) = 0.87–0.95. The bacterial-urine suspensions were allowed to grow in presence or absence of Mitomycin C, a compound that causes prophage induction through bacterial DNA damage18. Supernatants were separated from the pellets by centrifugation, concentrated using poly(NIPAm/AA) particles and plated using soft agar on a bacterial lawn. For both E. coli B and UPEC, the number of plaques detected in the bacterium cultured in the presence of UTI urine was higher than non-UTI urine (Fig. 6B–I, Supplementary Data 8). Mitomycin C caused the number of plaques to increase more than two-fold in all conditions. (Fig. 6B-I). The bacterial pellet was subjected to DNA extraction, and RT-PCR was performed for the following genes: Recombinase A protein and Recombinase A putative gene; xerC and xerD integrases/recombinases, Transposase, and CysG (housekeeping gene). These genes are responsible for integration, excision and induction of prophages and were chosen to monitor the activity of temperate phages. The activity of the insertion/excision machinery was higher in E. coli and UPEC spiked in UTI urine than in non-UTI urine (Fig. 6J, Supplementary Data 9). Mitomycin C treatment induced an increase in the insertion/excision genes when the bacteria were incubated with non-UTI urine and a slight decrease when bacteria were incubated with UTI urine (Fig. 6J). Overall, these data collected using model organisms E. coli B and UPEC CFT073 suggest (Supplementary Table 4) the presence of a higher number of temperate phages and a more robust and active recombination system when the organisms were grown in presence of UTI urine, presenting with greater competition due to a greater number of bacteria (OD600 = 0.28–0.31), than non-UTI urine, carrying no bacteria (OD600 = 0).
A Experimental pipeline included E. coli model organism incubation with UTI and non-UTI human urine in presence or absence of Mitomycin C, a prophage inducer. Cells were separated from the supernatant via centrifugation and both fractions were analyzed via plaque assay or real-time PCR (rt-PCR). Created in https://BioRender.com. B Plaque numbers were higher in presence of UTI urine than non-UTI urine and increased more than two-fold after Mitomycin C induction. Samples in the y-axis are labeled with the following codes: U = UTI urine, CFT073 = E. coli CFT073, MitoC = Mitomycin C induction, N = non-UTI urine, E.coli = E. coli B. For example, U-CTF073-MitoC = E. coli CFT073 cultured in presence of UTI urine and Mitomycin C induction. The mean and standard deviation were calculated on 7 replicates. C–E Digital camera picture of example plaques. Scale bar = 2 mm. F–I Optical microscope pictures of example plaques (4X magnification). Scale bar = 1 mm. J rt-PCR results show higher activity of the integration/excision/transposition machinery in E. coli B and UPEC spiked in UTI urine than in non-UTI urine. Samples in the x-axis are labeled with the following codes: U = UTI urine, CFT073 = E. coli CFT073, MitoC = Mitomycin C induction, N = non-UTI urine, E. coli = E. coli B. For example, U-CTF073-MitoC = E. coli CFT073 cultured in presence of UTI urine and Mitomycin C induction. The mean and standard deviation were calculated on 7 replicates.
Discussion
In the past, phage therapy failure was postulated to be caused by a variety of bacteria host-mediated anti-phage resistance mechanisms (e.g., gene mutations, CRISPRs, restriction-modification). In the present study, we used functional proteomic and real-time PCR analysis of UTI patient urine and bacteria model organisms to investigate the relevance of lysogeny in human infection (Fig. 7). Do phage populations in diverse microbiomes shift to cooperation with their prey when overwhelmed with the prey abundance? Our human data, put in perspective by comparison with phage-bacteria warfare in the aquatic environment4, raises the question: from a human health perspective, are temperate phages part of the problem and not the defenders we wished them to be? The answer to this question will greatly impact on future strategies for successful phage therapy.
When uropathogen bacterial numbers are low, in absence of symptomatic infection, the preferential phage lifestyle is lytic. When the bacterial numbers are high in presence of UTI, the phage population switches to a higher proportion of phages capable of lysogenic lifestyle. This switch supports both phage and bacterial fitness. During lysogeny, phages engage in symbiotic relationship with the bacterial host, whereby they express proteins that help bacterial fitness. Created in https://BioRender.com.
Temperate phage proportion increases as a possible bacterial fitness advantage
Although contrary to traditional models and assumptions19, a switch to lysogeny in high-density microbial communities has been recently postulated by Knowles and colleagues in diverse aquatic ecosystems4. Knowles et al. proposed that lysogeny is a resistance mechanism that could explain the lower virus to microbe (VTM) ratio observed in high-density bacterial communities. Lysogeny, in fact, confers superinfection immunity to the bacterial host4.
It must be noted that the model theorized by Knowles et al. has been widely discussed5. According to Weitz et al.5, three main pieces of empirical evidence are lacking to fully understand and model the relevance of lysogeny in diverse microbiomes: 1) rigorous simultaneous measurements both inside and outside the cell, 2) relative proportions of temperate and virulent phages, and 3) rates of prophage integration and induction. Evidence is also lacking for the model generalization to non-coral reef environments5.
In line with the ongoing discussion4,5, we provide herein empirical evidence related to the diverse microbiome of the human urinary tract. Our data include 1) estimates of the correlation between phage proteins and bacterial proteins, which is a proxy for the VTM ratio, 2) estimates of the relative proportions of temperate and virulent phages, 3) rates of prophage integration and induction, and 4) simultaneous data collection inside and outside the cells in E. coli/urine models. Further discussion follows for each of these points:
-
1.
Our proteomic data (Fig. 2) confirms a relative decrease of phage proteins with increase bacterial host density (log scale linear regression slope from 0.65-0.88), with linear regression slope values within the range of published reports of phage bacteria ratios in aquatic systems (slopes 0.2–0.94). We propose to consider this ratio as a functional proxy for the commonly used virus to microbe ratio4.
-
2.
To estimate the relative proportion of productive temperate and virulent phages, we used a published BACterioPHage LIfestyle Predictor (BACPHLIP) and proteomics data to show that UTI patients are more likely to have temperate phages than non-UTI controls (odd ratio 1.47, with a 95% confidence interval of 1.11–2.11, and a Fisher exact test p-value of 0.03, Fig. 2). BACPHLIP is a machine learning tool that predicts the lifestyles of bacteriophages (temperate or virulent) using conserved protein domains. BACPHLIP achieved high prediction accuracy, reaching 98.3% on a testing group of phages that were not used for training and performed better than existing methods14.
-
3,4.
Rates of prophage integration and induction were studied using gene expression analysis and plaque assays of model systems composed by laboratory strains E. coli B and E. coli CFT073 spiked in UTI and non-UTI human urine. Results confirmed a larger number of mitomycin C-induced prophages and a robust activity of the DNA recombination system in UTI urine (Fig. 6). The following genes involved in prophage integration, excision, and transposition were analyzed: 1) RecA, which mediates cleavage of the lytic gene repressor CI thus facilitating lytic genes expression20, 2) XerC and XerD, which are recombinases required for phage integration and excision21, and 3) Transposase, which is responsible for cleavage of a specific insertion sequence and its movement to another part of the genome22. These experiments showed that the recombination system in the UTI milieu is more active and robust and that UTI bacteria induce greater exchange of genetic material, possibly to enhance population fitness and genetic diversity. The gene expression analysis complemented and confirmed the information obtained from the urine proteomic analysis. It was noted that recombinase genes had lower abundance after mitomycin C treatment of the bacteria spiked in UTI urine. This finding is consistent with previous studies that show recombinases, and in particular, XerC, being down-regulated late in prophage induction23. Our proteomic analysis identified proteins involved in the phage excision and integration mechanism thus providing a link between the in vitro and in vivo systems. For example, we identified a Mu-like cryoconite phage AB09 YqaJ-like viral recombinase (Supplementary Data 2), which is an exonuclease involved in red recombination, a mechanism of homologous recombination of lambda and other temperate phages24,25,26 by supporting the digestion of linear double-stranded DNA in a Mg2+-dependent reaction27.
The literature suggests that lysogeny can have important consequences for the bacterial host, including remodeling the bacterial genome, altering the pathogen physiology, facilitating the exchange of bacterial DNA, and giving the bacteria new molecular tools to promote its own survival28. The effects of phage lysogeny may therefore benefit both the phage and the bacterium, but be detrimental to the human pathogen-host. Prophages can contribute to the formation and stability of biofilms in vivo29,30 and can encode bacterial virulence factors31. Here we identified the Enterobacteria phage fiAA91-ss Cytolethal distending toxin (CDT) V C subunit (Supplementary Fig. 4, Supplementary Data 2), which has cytotoxic effects on mammalian cells by causing mammalian cell cycle arrest, DNA damage, and cellular distension32. Thus, Cdt contributes to bacterial survival and amplifies the pathogenicity of microorganisms31.
Temperate lifestyle as a possible phage fitness advantage
Beyond functioning as a bacterial defense against viral infection, the phage-temperate lifestyle is simultaneously a fitness advantage in the intra-phage competition33. Mathematical modeling and in vitro experiments showed that a temperate phage capable of alternating lytic and lysogenic lifestyles outcompetes a purely lytic phage, even if the lytic phage has superior life history traits, such as larger burst size, faster adsorption rate and infection rate33. Switching between the lytic and lysogenic lifestyles allows the temperate phage a greater flexibility to survive fluctuations in the bacterial host population density, and low host availability33. Lysogeny, Cheong et al. reason, can be an adaptive trait that emerged in response to oscillation in bacterial host populations induced by the virus itself or by other environmental factors33. Previous studies suggest that the human urinary tract is occupied by a significant proportion of temperate phages34,35,36. Recent metagenomic studies of the urinary phagome of UTI and non-UTI individuals determined a large prevalence of integrase genes that suggests abundance of phages with lysogenic life style34,35,36. The high prevalence of urinary temperate phages was previously explained by urinary tract acidic conditions, which are largely unfavorable to phage particle stability34.
Phage isolation from urine is low yield
Phages from urine were identified using the agar overlay method and electron microscopy (Fig. 5). Our experience in this study was that the isolation of phages from urine is a low-yield procedure likely due to a number of issues, including phage low abundance, unknown plaque growth conditions, and unusual plaque morphology. Small plaque size, size heterogeneity and instability can be due to the fact that plaque storage and development conditions have not yet been found37. However, turbid plaques38, plaques with turbid margins, and concentric rings of surviving and lysed bacterial hosts similar to Figs. 5 and 6 have been attributed to lysogenic behavior in the past37,38. To address the issue of low phage abundance, we have used a phage concentration affinity reagent that displayed a phage recovery efficiency of 82%.
Implications for UTI therapy: engineered lysogenic phages
It has been proposed that UTI is the result of urinary microbiome dysbiosis within the urinary tract ecosystem, rather than exogenous invasion by an individual strain34. Understanding the implications of the resident phage microbiome lifestyle in the UTI urinary microbiome population dynamics is crucial for devising the next generation of effective treatment strategies. The preferential enrichment of lysogeny in high-density bacterial populations in human infections demonstrated in this study lays the foundation for exploiting lysogeny for therapeutical purposes. By engineering designer prophages, it may be possible to disrupt the virulence, antibiotic resistance, and biofilm formation of UTI-causing bacteria, leading to improved treatment outcomes. Certain prophage genes, frequently genes that contribute to bacterial fitness, are arranged in self-contained units of gene expression (morons) that can be activated and expressed independently while the prophage is in a dormant state7,39. Prophages could be engineered to produce bacteriocins40, for example, bacterial cell wall hydrolases, colicins, or pore-forming toxins, thereby causing bacterial death. Such engineered phages could be used to develop precision antimicrobial treatments, which could potentially prevent the complications associated with broad-spectrum antibiotics, such as antibiotic-induced microbiome dysbiosis40. The design of a prophage targeting therapy must address long-term outcomes and evolutionary dynamics, given the fact that over time the prophage genome can mutate, mediate horizontal gene transfer, or remain integrated into the bacterial genome41.
Antibiotics can play a significant role in the process of prophage excision. Studies have shown that certain antibiotics, such as mitomycin C, will induce a DNA repair response in bacteria, known as the SOS response. This response activates the excision of pro-phages from bacterial genomes and phage induction, triggering the phage switch from a temperate to a virulent lytic lifestyle. Research has demonstrated phage-antibiotic synergy; in particular, the sequential treatment of biofilms with phages followed by antibiotics is particularly effective in removing bacteria42,43. Such a combination approach may work because it reduces the frequency of the lysogenic cooperation switch.
Limitations and future directions
This study has several limitations. Even if the number of patients (N = 30) is limited, it yielded a large amount of proteomic information. Phage and bacterial proteins involved in all known defense mechanisms and life cycle phases were identified. The relatively small number of patients, however, might not fully capture the full range of variability within and between patients. By including samples from two countries, we aimed to capture some geographical diversity. Future studies including larger study sets, longitudinal studies over time and different sampling modalities will strengthen the generalizability of the results collected in this study.
Human urinary phages are largely unknown. Previous genomic studies of human urinary tract phages demonstrated that a large proportion ( > 80%) of isolated genomic sequences were unknown34. Since our proteomic approach is based on database matching, the scarce knowledge of human urinary phage genomic sequences limits the possible peptide identifications. As our collective knowledge of human urinary phage increases and more extensive databases are compiled, the number of peptide identifications will increase. Rapidly developing informatics tools to assist de novo sequencing peptides will also increase the yield of peptide identification44.
Proteomic analysis provided a large number of peptide identifications. To confirm the functional role of detected proteins and proposed phage lifestyle, in vitro validation studies, genomic and bioinformatic analyses are warranted.
Conclusions
These data reveal previously unknown evidence to support a shift from competition to cooperation of phages with pathogenic bacteria during human UTI development. This information can be leveraged in the future to identify novel therapeutic strategies for UTIs, including phage therapy and microbiome manipulation to enhance human health. The principle of a population lysogenic enrichment at high bacterial density is generalizable to other human bacterial pathogen systems. Future strategies for phage therapy should employ engineered lysogenic phages as a Trojan Horse.
Methods
Patient sample collection
Urine samples were collected from (Chesapeake IRB approval number Pro00008518) patients affected by chronic urinary tract infection (UTI) from the Country of Georgia (CoG, N = 10), non-UTI volunteers from the CoG (N = 10), and non-UTI controls from the United States (N = 10). Informed consent was obtained when enrolling participants in the study. All ethical regulations relevant to human research participants were followed. The study protocol was approved by the George Mason University Institutional Review Board (IRB) and by the Ethics Committee of the University of Georgia. In the CoG, patients were clinically diagnosed of UTI according to guidelines described by Gupta et al.45. The US non-UTI controls were patients suspected of harboring Borreliosis who lived in geographic regions at high risk for tick-borne illnesses, including New England, Northern Virginia, Maryland and Washington DC (clinic: Hope McIntyre, MD, Maryland). US non-UTI patients were considered to have late-stage Borreliosis according to the guidelines of the Infectious Disease Society of America (IDSA)46 if: 1) they were previously diagnosed for Lyme disease based on a positive two-tiered serology algorithm recommended by the CDC, and presence of an erythema migrans rash, b) their Borreliosis symptoms ameliorated following antibiotic treatment, and c) patients presented with fatigue, musculoskeletal pain, or cognitive impairment according to the IDSA criteria at the time of urine collection. Spot urine was collected in standard sterile wide-mouth urine collection devices (Thermo Scientific) and promptly frozen at -80°C. Samples were shipped in dry ice to George Mason University, where they were processed for analysis. This study included 30 volunteers who donated urine under informed consent. Patient samples were collected in the Country of Georgia (CoG) from patients who were diagnosed with primary urinary tract infection before antibiotic treatment (UTI, N = 10) and from patients who did not have UTI (N = 10). A set of 10 patients who had signs and symptoms of a non-UTI bacterial infection (Borreliosis) donated urine in the United States. Borreliosis patients were chosen as non-UTI bacterial infection controls because Borrelia doesn’t reside in the human urinary tract, Borrelia infection is paucibacillary, and Borrelia’s taxonomy separation from UTI pathogens supports the lack of common phages. Clinical and demographic information of enrolled patients is reported in Table 1. The median age was 51.5 years (min = 2, max = 81), gender distribution was female = 17, male = 13. No association was found between the age of patients and their clinical status (UTI versus non-UTI, t-test p = 0.85).
Affinity hydrogel particle preparation
Poly(N-isopropylacrylamide (NIPAm)-co-Allylamine (AA)) particles were prepared via precipitation polymerization15. NIPAm (0.89 g, 7.83 mmol) and N, N’-methylenebisacrylamide (BIS, 0.042 g, 0.27 mmol) were dissolved in 30 mL of water and then passed through a 0.2 μm nylon membrane filter. The solution was purged with nitrogen for 15 min at room temperature with a medium stirring rate. AA (Sigma, 0.051 g, 0.90 mmol) was added; the solution was purged with nitrogen for 15 min and then heated to 70 °C. KPS (0.0070 g, 0.025 mmol) was dissolved in 1.0 mL of water and was added to the solution to initiate polymerization. The reaction was maintained at 70 °C under nitrogen for 3 h. The reaction was subsequently allowed to cool to room temperature and stirred overnight. Particles were washed five times by centrifugation (19 rcf, 50 min, 25 °C, Avanti JXN-26 Backman Coulter high-speed centrifuge) with water in order to eliminate the unreacted monomer and then resuspended in 30 mL of H2O. Poly(NIPAm/AA) particles were used to precipitate bacteriophages from urine. In order to capture the urinary proteome, hydrogel particles were functionalized with Reactive Blue 221 poly(NIPAm/AA/RB221) as follows15:1.325 g of Na2CO3 (Sodium Carbonate) was dissolved in 100 ml of MilliQ water. 3 g of the Reactive Blue 221 dye was dissolved in the solution and stirred for 30 min. The solution was then filtered twice using the EMD Millipore™ Stericup™ Sterile Vacuum Filter Units. After filtration, 100 mL of the poly(NIPAm/AA) particles were mixed with the dye solution and stirred for 24 h. Finally, the suspension was washed by centrifugation (19 rcf for 45 min for 5–7 cycles) to eliminate the unbound dye.
Urinary proteome affinity capture
Dipstick urinalysis was performed on all the urine samples using a Multistix 10 SG reagent strip. Urine was then divided into 40 mL aliquots. A preliminary centrifugation step for 15 min at 3.5 rcf was performed to eliminate cryoprecipitates and cellular debris. The acidity of the urine was adjusted to pH~6 using 10 mM hydrochloric acid. Chicken lysozyme (10 ng) was spiked in each sample as an internal control. Urine samples were then enriched with 200 µL of the poly(NIPAm/AA/RBB) particles (10 mg/mL) and incubated for 45 min at room temperature. After that, the urine samples were centrifuged at 19 rcf (Beckman Avanti JXN-26 Centrifuge) for 45 min. The pellet was then washed twice with 1 mL of 18 MΩ cm water and centrifuged at 16,100 × g for 20 min. Supernatant was discarded and the particle pellet was resuspended in 20 µL of elution buffer solution obtained by dissolving 1 mg of RapiGest (Waters Corporation) in 190 µL of 50 mM Ammonium bicarbonate additioned with 10 µL of tris(2-carboxyethyl)phosphine (TCEP, Thermo Scientific). The elution buffer was allowed to incubate for 20 min at room temperature. Samples were finally centrifuged at 16.1 rcf for 20 min. Eluates were processed for mass spectrometry.
Mass spectrometry analysis
The Thermo Scientific Orbitrap Exploris 480 was operated in a data-dependent mode consisting of one full MS scan (60,000 resolution) from 300 Da to 1500 Da followed by up to 20 MS/MS acquisitions of the most abundant precursor ions dynamically selected by Top Speed and fragmented by higher energy collisional dissociation (HCD) using a normalized collision energy of 27%. Peptides were separated using a reversed-phase PepMap RSLC 75 μm i.d. × 15 cm long with 2 μm, C18 resin LC column (ThermoFisher Scientific, Waltham, MA, USA). The mobile phase was 0.1% aqueous formic acid (mobile phase A) and 0.1% formic acid in 80% acetonitrile (mobile phase B). After sample injection, the peptides were eluted by using a linear gradient from 5% to 50% B over 15 min and ramped to 100% B for an additional 2 min. The flow rate was set at 300 nL/min. Peptide Monoisotopic Precursor Selection” and “Dynamic Exclusion” (8 s duration), were enabled, as was the charge state dependency so that only peptide precursors with charge states from +2 to +4 were selected and fragmented by HCD. Precursor mass tolerance was set at 2 ppm, and fragment ion tolerance to 0.05 Da. Data was analyzed allowing for variable oxidation ( + 15.9949 Da) of methionine, and carbamidomethylation of cysteines ( + 57.0215) as a fixed modification. Tandem mass spectra were searched against the Homo sapiens, uropathogenic Escherichia coli (UPEC), Escherichia coli 0157, Escherichia coli O25b, Enterobacter cloacae, Enterococcus faecalis, Staphylococcus aureus, Streptococcus agalactiae, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Klebsiella pneumoniae IS43UP, and Bacteriophages NCBI and Uniprot databases with the Proteome Discoverer 3.1 software using tryptic cleavage constraints. The following parameters were used: Xcorr versus charge 1.9, 2.2, 3.5 for 1 + , 2 + , 3+ ions respectively; ΔCn > 0.1; probability of randomized identification e0.01, and 1% false discovery rate (FDR) was used.
Phage isolation from urine using the overlay technique
Bacteriophages were isolated from urine with the agar overlay method47. In brief, one mL of urine was mixed with 2.5 ml molten (45 °C) Luria Bertani (LB) (Becton Dickinson, Erembodegem, Belgium), containing 0.7% top agar (Bacto agar, Becton Dickinson), and a suspension of bacteriophage sensitive bacteria (end concentration of 108/mL) in sterile 14 ml tubes (Falcon, Becton Dickinson). This mixture was plated in triplicate onto 90 mm diameter Petri dishes (Plastiques Gosselin, Menen, Belgium) filled with a bottom layer of 1.5% LB agar and incubated for 18–24 h at 37 °C.
Transmission electron microscopy
A single plaque was picked and suspended in TM buffer (50 mM Tris–HCl, 10 mM MgCl2, pH 7.4). Around 3 µL of purified bacteriophage suspension was gently placed on glow-discharged carbon-coated 300 mesh copper grids. After about 1 min, the remaining solution on the grids was wicked away with the help of a filter paper. The grid was then stained with 2% (wt/vol) uranyl acetate and air-dried. The negatively stained phage particles were visualized with a Jeol JEM 1400 Flash electron microscope at an operating voltage of 100 kV48. Virus particle dimensions were measured using the ImageJ computer program (version 1.53e, https://imagej.net/ij/), with the software scale set on the scale bar obtained from the electron micrographs48.
Phage precipitation using affinity hydrogel particles
In order to determine the capability of affinity hydrogel particles to capture phages, poly(NIPAm/AA) affinity particles were mixed with T3 phage suspensions in LB, precipitated by centrifugation and then plated using the double agar overlay method. More in detail, 250 µL of T3 phage suspensions (107 and 5 * 104 pfu/mL) in sterile Luria Bertani (LB) were incubated with 200 µL of poly(NIPAm/AA) affinity particles (10 mg/mL) for 45 min at room temperature. Particles were collected by centrifugation at 16.1 rcf for 20 min, washed with phosphate buffer saline (PBS) and resuspended in 100 µL of sterile water. The poly(NIPAm/AA) particles were plated using the double agar overlay method and compared to 100 µL of T3 phages (107 and 5 * 104 pfu/mL) and naïve poly(NIPAm/AA) particles. All samples were mixed with LB broth as a diluent and added to 200 µL of a host bacterial strain suspension (end concentration of 108/mL). Samples were then added to a tube containing 3 mL of 0.5% LB/agar (top agar) prewarmed at 37 °C, mixed well and poured over prewarmed 2% LB/agar plates. The top agar was allowed to harden, then plates were inverted and incubated at 37°C for 4 h to overnight. Plaques developed after 4 h of incubation. The recovery efficiency of the affinity particles was calculated using the formula \(E=\frac{{{PFU}}_{2}}{{{PFU}}_{1}}\frac{{V}_{2}}{{V}_{1}}=\,\frac{{{PFU}}_{2}}{{{PFU}}_{1}\cdot {C}_{f}}\) where E is the recovery efficiency, PFU1 and PFU2 are the plaque-forming units of the initial T3 phage suspension and of the affinity particles, respectively, and Cf is the concentration factor given by the ratio of the initial volume V1 divided by the final volume V2.
Determination of prophage induction, excision, integration, and transposition rates
E. coli B (American Type Culture Collection, ATCC catalog number 11303) and E. coli CFT073 [WAM2267] UPEC strain (ATCC catalog number 700928) was spiked in 5 mL of urine from UTI and non-UTI human participants diluted 1:1 with LB broth at a concentration of 10^8 cells/mL. Cultures were allowed to grow at 37 °C for 6 h, and then mitomycin C was added to the cultures at a concentration of 0.1 μg/mL and allowed to incubate with the culture for additional 6 h at 37 °C. The cultures were subjected to centrifugation (10,000 rpm, 5 min, room temperature) and the supernatant was mixed with 100 μL of poly(NIPAm/AA) particles to capture the phages and allowed to incubate for one hour at room temperature. Particles were then collected and washed with PBS via centrifugation (16.1 rcf, 10 min, room temperature), resuspended in 100 μL of DI water and on plated on E. coli B and UPEC lawns on a soft agar to check for plaque visualization. The bacterial pellet was subjected to gene expression analysis. DNA was isolated from the bacterial pellet using phenol:chloroform:isoamyl alcohol (25:24:1) and RT PCR was performed for the expression of Recombinase A protein (AE014075.1 locus tag c3253), Recombinase A putative gene (locus tag c0291), xerC (locus tag c4732) and xerD (locus tag c3474) integrase/recombinase (locus tag c1401), and T200_SALTY transposase (locus tag c0002). These genes are responsible for the exchange of genetic material and the integration of phage DNA, thus confirming the existence of prophages within the bacterial cells that are capable of temperate phage life cycle characterized by both lytic and lysogenic phases. CysG was used as a housekeeping49. The following primers were used: XerC Reverse 5’-AGC ATA TGG GTA GCG AAC GA, XerC Forward 5’-GTG ATG GGA AAA GGC AGC AA, XerDg Reverse 5’-5TC GGG CTG ACA ATG AGT GAT, XerDg Forward 5’-ACA CAC CAT TCA AC AGC CAC, XerDpr Reverse 5’-ACA CAC CAT TCA ACA GCC AC, XerDpr Forward 5’-TCG GGC TGA CAA TGA GTG AT, RecAg Reverse 5’-GAA GAA CAA AAT CGC TGC GC, RecAg Forward 5’-CAT TCG CTT TAC CCT GAC CG, RecApr Reverse 5’-CAT TCG CTT TAC CCT GAC CG, RecApr Forward 5’-GAA GAA CAA AAT CGC TGC GC, Transposase Reverse 5’-TCC TGT ATC TTC GCC GTG TT, Transposase Forward 5’-GAG ATC CCG CCC AAA ATG AG, CysG Reverse 5’-ATG CGG TGA ACT GTG GAA TAA ACG, CysG Forward 5’-TTG TCG GCG GTG GTG ATG TC.
Statistics and reproducibility
Heatmaps and two-way hierarchical clustering were performed with Proteome Discoverer 3.1. Venn diagrams, boxplots50, t-tests, limma analysis, lasso analysis, Pearson correlation analysis, and odd ratio calculations were performed using R software (https://www.r-project.org/).number of observations for the mass spectrometry analysis study was 30. Bacphlip analysis was conducted using the script deposited in GitHub14. Plate plaque experiments and real-time PCR were conducted using seven replicates. In both cases, the replicates were defined as bacterial aliquots derived from the same bacterial suspension, spiked into urine aliquots obtained from one participant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE51 partner repository with the dataset identifier PXD059932. This manuscript is accompanied by nine Supplementary Data files. Supplementary Data 1 contains mass spectrometry proteomic analysis results of T3 phage protein lysate spiked in human urine. Supplementary Data 2 contains bacteriophage mass spectrometry proteomic analysis results of urine samples collected from UTI and non-UTI patients. Supplementary Data 3 contains the uropathogenic bacteria mass spectrometry proteomic analysis results of urine samples collected from UTI and non-UTI patients. Supplementary Data 4 contains Homo sapiens mass spectrometry proteomic analysis results of urine samples collected from UTI and non-UTI patients. Supplementary Data 5 contains phage annotations predicted by Bacphlip. Supplementary Data 6 contains the results of phage protein abundance limma analysis. Supplementary Data 7 contains the results of bacterial protein abundance limma analysis. Supplementary Data 8 contains plaque numbers, supporting Fig. 6B. Supplementary Data 9 contains relative gene expression values, supporting Fig. 6J.
Code availability
BACPHLIP14 is freely available on GitHub (https://github.com/adamhockenberry/bacphlip).
References
Al-Anany, A. M. et al. Phage therapy in the management of urinary tract infections: a comprehensive systematic review. Phage4, 112–127 (2023).
Ling, H. et al. Recent advances in bacteriophage-based therapeutics: Insight into the post-antibiotic era. Acta Pharm. Sin. B 12, 4348–4364 (2022).
James, C. E. et al. Lytic activity by temperate phages of Pseudomonas aeruginosa in long-term cystic fibrosis chronic lung infections. ISME J. 9, 1391–1398 (2015).
Knowles, B. et al. Lytic to temperate switching of viral communities. Nature 531, 466–470 (2016).
Weitz, J. S., Beckett, S. J., Brum, J. R., Cael, B. B. & Dushoff, J. Lysis, lysogeny and virus-microbe ratios. Nature 549, E1–E3 (2017).
Campbell, A. The future of bacteriophage biology. Nat. Rev. Genet 4, 471–477 (2003).
Fortier, L. C. & Sekulovic, O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 4, 354–365 (2013).
Yang, D. et al. A temperate. Virulence 13, 137–148 (2022).
Nepal, R. et al. Prophages encoding human immune evasion cluster genes are enriched in. Microb. Genom. 7 https://doi.org/10.1099/mgen.0.000726 (2021).
Magni, R., Luchini, A., Liotta, L. & Molestina, R. E. Analysis of the Babesia microti proteome in infected red blood cells by a combination of nanotechnology and mass spectrometry. Int J. Parasitol. 49, 139–144 (2019).
Magni, R. et al. Evaluation of pathogen specific urinary peptides in tick-borne illnesses. Sci. Rep. 10, 19340 (2020).
Steinberg, H. et al. Diagnosis of toxoplasmosis reactivation in hiv patients in urine using nanoparticle technology. Am. J. Tropical Med. Hyg. 95, 359 (2017).
Steinberg, H. E. et al. Detection of toxoplasmic encephalitis in HIV positive patients in urine with hydrogel nanoparticles. PLoS Negl. Trop. Dis. 15, e0009199 (2021).
Hockenberry, A. J. & Wilke, C. O. BACPHLIP: predicting bacteriophage lifestyle from conserved protein domains. PeerJ 9, e11396 (2021).
Tamburro, D. et al. Multifunctional core-shell nanoparticles: discovery of previously invisible biomarkers. J. Am. Chem. Soc. 133, 19178–19188 (2011).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Seed, K. D. Battling phages: how bacteria defend against viral attack. PLoS Pathog. 11, e1004847 (2015).
Kohm, K. & Hertel, R. The life cycle of SPβ and related phages. Arch. Virol. 166, 2119–2130 (2021).
Suttle, C. A. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol 5, 801–812 (2007).
Atsumi, S. & Little, J. W. Role of the lytic repressor in prophage induction of phage lambda as analyzed by a module-replacement approach. Proc. Natl. Acad. Sci. USA 103, 4558–4563 (2006).
Huber, K. E. & Waldor, M. K. Filamentous phage integration requires the host recombinases XerC and XerD. Nature 417, 656–659 (2002).
Styles, K. M., Locke, R. K., Cowley, L. A., Brown, A. T. & Sagona, A. P. Transposable element insertions into the escherichia coli polysialic acid gene cluster result in resistance to the K1F bacteriophage. Microbiol. Spectr. 10, e0211221 (2022).
Osterhout, R. E., Figueroa, I. A., Keasling, J. D. & Arkin, A. P. Global analysis of host response to induction of a latent bacteriophage. BMC Microbiol. 7, 82 (2007).
Yang, M. et al. Genomic characterization and distribution pattern of a novel marine OM43 phage. Front. Microbiol. 12, 651326 (2021).
Gilcrease, E. B. & Casjens, S. R. The genome sequence of Escherichia coli tailed phage D6 and the diversity of Enterobacteriales circular plasmid prophages. Virology 515, 203–214 (2018).
Caldwell, B. J. & Bell, C. E. Structure and mechanism of the Red recombination system of bacteriophage λ. Prog. Biophys. Mol. Biol. 147, 33–46 (2019).
Chen, W. Y., Ho, J. W., Huang, J. D. & Watt, R. M. Functional characterization of an alkaline exonuclease and single strand annealing protein from the SXT genetic element of Vibrio cholerae. BMC Mol. Biol. 12, 16 (2011).
Feiner, R. et al. A new perspective on lysogeny: prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 13, 641–650 (2015).
Carrolo, M., Frias, M. J., Pinto, F. R., Melo-Cristino, J. & Ramirez, M. Prophage spontaneous activation promotes DNA release enhancing biofilm formation in Streptococcus pneumoniae. PLoS One 5, e15678 (2010).
Liu, Y. et al. Prophage phiv205-1 facilitates biofilm formation and pathogenicity of avian pathogenic Escherichia coli strain DE205B. Vet. Microbiol. 247, 108752 (2020).
Allué-Guardia, A., García-Aljaro, C. & Muniesa, M. Bacteriophage-encoding cytolethal distending toxin type V gene induced from nonclinical Escherichia coli isolates. Infect. Immun. 79, 3262–3272 (2011).
Lai, Y. R. et al. From DNA damage to cancer progression: potential effects of cytolethal distending toxin. Front. Immunol. 12, 760451 (2021).
Cheong, K. H., Wen, T., Benler, S., Koh, J. M. & Koonin, E. V. Alternating lysis and lysogeny is a winning strategy in bacteriophages due to Parrondo’s paradox. Proc. Natl. Acad. Sci. USA 119, e2115145119 (2022).
Żaczek, M., Weber-Dąbrowska, B., Międzybrodzki, R. & Górski, A. Phage prevalence in the human urinary tract-current knowledge and therapeutic implications. Microorganisms 8 https://doi.org/10.3390/microorganisms8111802 (2020).
Miller-Ensminger, T. et al. Bacteriophages of the urinary microbiome. J. Bacteriol. 200 https://doi.org/10.1128/JB.00738-17 (2018).
Santiago-Rodriguez, T. M., Ly, M., Bonilla, N. & Pride, D. T. The human urine virome in association with urinary tract infections. Front Microbiol 6, 14 (2015).
Hopwood, D. A., Chater, K. F., Dowding, J. E. & Vivian, A. Advances in Streptomyces coelicolor genetics. Bacteriol. Rev. 37, 371–405 (1973).
Hammerl, J. A. et al. Host range, morphology and sequence analysis of ten temperate phages isolated from pathogenic. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23126779 (2022).
Taylor, V. L., Fitzpatrick, A. D., Islam, Z. & Maxwell, K. L. The Diverse impacts of phage morons on bacterial fitness and virulence. Adv. Virus Res 103, 1–31 (2019).
Heilbronner, S., Krismer, B., Brötz-Oesterhelt, H. & Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol 19, 726–739 (2021).
Marchi, J., Zborowsky, S., Debarbieux, L. & Weitz, J. S. The dynamic interplay of bacteriophage, bacteria and the mammalian host during phage therapy. iScience 26, 106004 (2023).
Jérôme, G. Prophage in phage manufacturing: is the risk overrated compared to other therapies or food? Antibiotics 9 https://doi.org/10.3390/antibiotics9080435 (2020).
Łusiak-Szelachowska, M. et al. Bacteriophages and antibiotic interactions in clinical practice: what we have learned so far. J. Biomed. Sci. 29, 23 (2022).
Zhang, J. et al. PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell Proteom. 11, M111.010587 (2012).
Gupta, K., Grigoryan, L. & Trautner, B. Urinary tract infection. Ann. Intern. Med. 167, ITC49–ITC64 (2017).
Donta, S. T. Issues in the diagnosis and treatment of Lyme disease. Open Neurol. J. 6, 140–145 (2012).
Merabishvili, M. et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4, e4944 (2009).
Howard, M. et al. A secretory form of Parkin-independent mitophagy contributes to the repertoire of extracellular vesicles released into the tumour interstitial fluid in vivo. J. Extracell. Vesicles 11, e12244 (2022).
Zhou, K. et al. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol. Biol. 12, 18 (2011).
McGill, R., Tukey, J. W. & Larsen, W. A. Variations of box plots. Am. Statistician 32, 12–16 (1978).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Acknowledgements
The research was supported by the Virginia Innovation Partnership Corporation CCF Award CCF23-0104-HE, the Beck Foundation, and George Mason University.
Author information
Authors and Affiliations
Contributions
M.A. performed experiments, data analysis, wrote and reviewed the manuscript, A.K. conceived the study, A.C. and H.M. procured and characterized the urine samples, L.T. conceived the study, N.C. performed experiments, provided expert advice, N.V, S.S.I. and E.N. conducted experiments, A.J.H. and R.P.A. conducted modeling and data analysis, W.Z. conducted experiments, B.B. conceived the study, conducted experiments and data analysis, wrote the paper, L.A.L. and A.L. conceived the study, analyzed the data, wrote and reviewed the manuscript. All authors have reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LAL and AL are coinventors in granted patents US 9,012,240 and US 8,497,137, related to the affinity particles. The patents are owned by the George Mason Research Foundation. Ceres Nanosciences licensed the rights of the patents. LAL and AL own shares of Ceres Nanosciences. The remaining authors declared no competing interests.
Peer review
Peer review information
Communications Biology thanks Dr Benoit Pons and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Karthika Rajeeve and Johannes Stortz.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Almosuli, M., Kirtava, A., Chkhotua, A. et al. Urinary bacteriophage cooperation with bacterial pathogens during human urinary tract infections supports lysogenic phage therapy. Commun Biol 8, 175 (2025). https://doi.org/10.1038/s42003-025-07598-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07598-8