Abstract
The removal of mesopredatory fishes by fishing may be a key factor driving outbreaks of crown-of-thorns starfish on coral reefs. Evidence for this idea has been derived from correlations between starfish densities and fishing pressure. However, dietary analyses using DNA, studies of the trophic role of mesopredatory fishes and experiments that have invoked threat responses suggest that outbreaks could also result from a trophic cascade driven, in part, by changes in the anti-predator behaviours of these fishes. If corroborated, this hypothesis could inform management decision-making, slowing the frequency of outbreaks and improving the health of coral reefs in the Anthropocene.
Similar content being viewed by others
Introduction
Coral reefs are one of the most threatened tropical ecosystems on Earth and recurrent outbreaks of the corallivorous crown-of-thorns starfish (hereafter CoTS; Acanthaster spp.) are a key process contributing to their decline. Outbreaks can contain millions of individual starfish that can deplete entire reefs of corals within short time scales (weeks–months)1,2. Since CoTS outbreaks were first documented in the late 1950’s and early 1960’s1, multiple non-exclusive hypotheses have been proposed to explain why they occur3. These can be divided into two general categories; firstly, outbreaks are caused by bottom-up processes of recruitment that feature high survivorship of larvae in the plankton and secondly, suggestions that a reduction in predation during the benthic phase of the life history of these starfish, a top-down process, is responsible for outbreaks3. In this Perspective, we examine top-down hypotheses in the light of new research emerging on the diet, role and behaviour of mesopredatory reef fishes. Synthesis of this work offers a plausible mechanism for the top-down control of CoTS outbreaks that involves both the direct and indirect consequences of mesopredator removal on coral reefs. We argue that the loss of the largest mesopredators has resulted in changes to the landscape of fear for smaller predatory species in reef environments that has in turn altered their trophic role and impact on populations of benthic invertebrates. We describe a roadmap to test this hypothesis, which, if corroborated, might offer tractable management options to increase the health of coral reef systems in the face of multiple anthropogenic threats.
Top-down or bottom-up?
The idea that top-down mechanisms can control CoTS outbreaks dates back to the 1960s4. These predator removal hypotheses state that the likelihood of outbreaks is driven by the loss of mesopredatory fishes and invertebrates via fishing. This concept has remained contentious for many decades because supporting evidence was either largely theoretical or anecdotal. Importantly, in the past, very few studies of mesopredators produced evidence that these fishes actually consumed CoTS4. The most obvious evidence for direct predation featured the giant triton (Charonia tritonis), a large gastropod known to consume adult CoTS3. However, the slow rate of food consumption and a preference for alternative prey by tritons made it unlikely that these could have an impact sufficient to avoid or reduce outbreaks3,4.
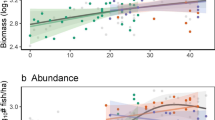
In the late 1970s and early 1980s, coral reef ecology underwent a paradigm shift that moved research from an emphasis on top-down processes that had dominated the field to one that focused on recruitment (a bottom-up process) as a key driver of population dynamics. Research on CoTS followed this trend, refocusing attention on planktonic processes and recruitment patterns as determinants of outbreaks. Studies suggested that outbreaks were the result of peaks in recruitment of young CoTS to benthic populations that were driven by high survivorship of larvae during the planktonic phase of the life history5,6. Nutrient enrichment of coastal water through pollution and coastal run-off has been argued to be an important contributor to planktonic survivorship (the nutrient enrichment hypothesis)7. On the Great Barrier Reef (GBR), where multiple outbreaks are a primary cause of coral declines over recent decades8, nutrient enrichment occurs through coastal run-off, and this has (in part) stimulated a focus on land management in adjacent terrestrial systems to improve the quality of water entering the reef9. However, since the 1980s there have been many observations of outbreaks of CoTS that are difficult to reconcile with the nutrient enrichment hypothesis. Notably, there have been outbreaks on coral reefs in the Red Sea and on isolated reefs in the Indian and Pacific oceans (e.g., the Chagos Archipelago, the Maldives, Micronesia, French Polynesia) where there was no apparent source of nutrient enrichment from terrestrial sources that might drive large year classes of CoTS larvae1. Additionally, the work of Dulvy et al.10 in the early 2000’s reignited the debate about the importance of top-down processes by documenting evidence of CoTS outbreaks along a gradient of fishing pressure on reefs in Fiji. They showed that where mesopredator density was reduced due to fishing, densities of CoTS increased by up to three orders of magnitude (Fig. 1 A, B). This result has now been supported by similar observations from two long-term (decadal) monitoring studies on the GBR, where removal of mesopredatory fishes by fishing was also correlated with increased outbreaks and densities of CoTS (Fig. 1C, D)11,12. Today, most researchers acknowledge the possibility that both top-down and bottom-up processes are likely to influence outbreaks13, but in the case of the predator-removal hypothesis, there is a lack of any obvious mechanism as to how this might occur—if mesopredatory fishes only rarely or never eat CoTS, how could they possibly regulate outbreaks?
In a study of 13 coral reefs of oceanic islands in Fiji, (A) shows the relationship between fishing pressure (as measured by people km–1 reef) and density of mesopredatory (piscivorous and invertivorous) fishes and, (B) shows the relationship between fishing pressure and average density of CoTS. Islands where no starfish were recorded are shown as grey symbols. Solid line is a linear regression that does not include grey symbols. Plots redrawn from Dulvy et al.10 C shows the occurrence of outbreaks of CoTS on mid-shelf reefs (where most outbreaks occur) on the Great Barrier Reef from 1994–2004. Reefs are categorised by management zoning in the Great Barrier Reef Marine Park (GBRMP) as open to fishing (red) or no-take marine protected areas (teal). Dark shaded bars show reefs with outbreaks, light shaded bars show reef with no outbreaks. Over a decade, only 20% of no-take mid-shelf reefs suffered outbreaks whereas outbreaks occurred on 75% of mid-shelf reefs open to fishing. Plot redrawn from Sweatman12. D Boxplots of model outputs showing effects of management zoning on likelihood of outbreaks of crown-of-thorns on 56 paired reefs, one open (red) and the other closed (green) to fishing spread across the Great Barrier Reef from 2006–2020. Plot redrawn from data in Kroon et al.11.
Mechanisms for the top-down control of CoTS outbreaks
Recent studies provide some answers to this question. Firstly, many fishes are now known to be capable of preying on CoTS, particularly during early stages of the life history (eggs, larvae, newly settled recruits and emergent juveniles) of the starfish4. Secondly, the development of DNA sampling of guts and faeces shows that these include mesopredatory species that are primary targets of fishing such as lethrinids and lutjanids14. However, the question of how often feeding on CoTS occurs, and if it could happen at a rate sufficient to control outbreaks, remains outstanding.
At the same time as the development of new approaches to investigate feeding, there has been a growing recognition of how fishing might affect the trophic dynamics of coral reefs as a whole and the role of mesopredators. In a study of coral reefs on the NW Shelf of Western Australia, Barley et al.15 found that on the Scott Reefs (North Scott, South Scott and Seringapatam reefs), where traditional fishing by Indonesian fishers targeted reef sharks (the largest reef mesopredator), the diet of several smaller teleost mesopredators contained significantly greater components of pelagic, high-energy prey including small fishes and squid. On the neighboring Rowley Shoals (Clerke, Imperieuse and Mermaid reefs) where reef sharks were protected and abundant, the diet of these same fishes was composed of a much larger proportion of benthic invertebrates, including asteroids (Fig. 2). This led to striking differences in body condition—some species of these mesopredators were 20% heavier for a given length on the Scott Reefs than individuals at the Rowley Shoals (Fig. 2). These results suggest that fishing not only removes mesopredators that prey on CoTS but also disrupts the trophic structure of the predatory food chain in a way that has implications for the population dynamics of both mesopredators and benthic invertebrates, including starfishes.
Diets are shown in pie charts as % abundance in gut contents and length-weight relationships are shown as log-scaled scatterplots. Pie charts and plots show results for the common mesopredatory teleosts, Lutjanus gibbus (A-C), L. decussatus (D-F) and L. bohar (G-I) for the Scott Reefs (fished) and the Rowley Shoals (unfished marine protected area). In pie charts, components of the diet that are pelagic in origin are coloured in shades of blue, benthic components are tan shades. Arrow shows asteroids as a component of the diet. Solid lines in plots are least squares regression relationships between log-scaled data. Data points are coloured red from the Scott Reefs and green from the Rowley Shoals. Data replotted from Barley et al.15.
This conclusion is now supported by experimental studies that examine the response of these smaller mesopredatory fishes to predatory threat. As it is logistically difficult and unethical to remove or exclude the largest mesopredatory species from a reef system, these experimental studies have invoked predator threat using life-size models. In a recent study on the GBR, Lester et al.16 showed that models of blacktip sharks (Carcharhinus melanopterus) reduced the distance that mesopredators were willing to travel from shelter to forage. Importantly, this phenomenon was much stronger in the water column above the reef than on the seafloor (Fig. 3), implying that these fishes are more wary of, and thus likely to be more vulnerable to, predators when foraging in the open water column than on the benthos. Field evidence for this idea comes from baited underwater video surveys of reef fish communities across northern Australia. Lester et al.17 found that the appearance of reef sharks around bait bags in the video invoked predictable anti-threat behaviours in mesopredatory reef fishes whereby they descended from the water column to shelter among the coral benthos (Fig. 4).
The use of models to invoke anti-predator behaviour has a long history in ecology, particularly in studies of avian communities. Recent work has expanded this approach into coral reef environments using models of reef sharks and smaller teleost predators to examine the response of herbivorous and mesopredatory species to different scenarios of predatory threat. Lester et al.16 examined the willingness of mesopredatory lutjanid, serranid and lethrinid fishes to venture onto the sand beside and into the water column above coral patches to feed on fish baits. Baits were deployed at three distances along a horizontal axis on the seabed perpendicular to the reef and in a vertical axis in the water column above the reef. Horizontal and vertical deployments occurred separately. Predator threat was manipulated by the presence of life-size models of a common reef shark, the reef blacktip (Carcharhinus melanopterus, a predator and/or lethal competitor) and a model of a small, non-threatening mesopredator (a coral trout, Plectropomus sp., a competitor) and object controls (silhouettes). Crosses and lines show mean time taken to first bite on the baits on a horizontal axis (blue) and a vertical axis (tan) at different distances from the reef (A). As the distance from the patch reef increased, it took longer for mesopredators to initiate feeding and the amount of time increased when the model reef shark was deployed. Importantly, the average time taken for feeding to commence above the patch reef in the presence of the shark model was almost twice the amount taken over the sand at just under half the equivalent distance from the reef. This showed that these smaller mesopredators were far more sensitive to the threat of predation away from shelter in vertical than horizontal axes—effectively defining an elongated hemisphere of risk around the patch reefs (B). Lester et al.16 argued that on the sand, mesopredators were protected from attack from below whereas above the reef, these fishes were particularly vulnerable, given the placement and sensitivity of their sensory systems (notably vision). Data sets re-plotted from Lester et al.16. Image artwork credit: Dean Tysdale.
Lester et al.17 surveyed fish and shark communities using stereo-baited underwater video systems (stereo-BRUVS42) at the Rowley Shoals and the Scott Reefs, two adjacent sets of large, atoll-like reefs on the edge of the continental shelf off the coast of northern Western Australia. Sharks are abundant at the Rowley Shoals, which have been a no-take marine protected areas for over 30 years, whereas they are very rare at the Scott Reefs, which are open to shark fishing by Indonesian fishers using traditional harvest techniques (longlines). BRUVS were used to compare behaviours of teleost mesopredators (lutjanids, lethrinids serranids) at each of these reef systems. Lester et al.17 showed that common species spent more time in the water column at the Scott Reefs where sharks were rare (A, B), a result consistent with experimental studies of responses of these species to predation threat (see Fig. 3). Bargraphs show average percentage time spent in water column +/− S.E. for Lutjanus bohar (A) and L. gibbus (B). Points show raw data. For L. bohar, plot also shows relationship between mean relief and the percentage of time spent in midwater where the solid line indicates the estimated smoothing curve and the shaded area indicates the S.E. of the estimate. For L. gibbus, the plot also shows relationship between number of bites made by sharks of the bait bag of the BRUVS and the percentage of time spent in midwater by this species, where the solid line indicates the estimated smoothing curve and the shaded area indicates the S.E. of the estimate. The response to predation threat was mediated by both environmental context and behaviour of reef sharks; the time spent in the water column by Lutjanus bohar increased when the benthos was complex and there was a large amount of cover (A), whereas L. gibbus spent less time in midwater once sharks began actively biting on the bait bag of the camera systems (B). Data sets replotted from Lester et al.17. Image artwork credit: Dean Tysdale.
A common criticism of studies that suggest that smaller mesopredators react to reef sharks and large serranids as a predatory threat is that there is relatively little evidence that such large species actually consume these reef fishes (e.g18,19.), mostly because few gut content studies of sharks have identified mesopredatory fishes as prey items19,20. As shown by the debate about consumption of CoTS by fishes, resolution of this argument awaits better studies of gut contents of sharks that use techniques such as DNA to identify food items. In any event, whether sharks directly target fish mesopredators as prey is largely irrelevant in the context of threat-adverse behaviour. As the experiments and field studies cited above show, teleost mesopredators will alter their behaviour to the presence of threat in ways that are consistent with changes in the trophic niche space they occupy in reef systems with and without sharks. This reaction does not require that sharks focus on other smaller mesopredatory reef fishes as prey: rather, it is more likely that the response by these smaller fishes is to a potentially lethal competitor21. In many other ecosystems, larger or apex predators will often attack and kill smaller mesopredators to reduce competition and this evokes threat-adverse behaviours in these smaller predators. The terrestrial ecological literature is replete with examples of this behaviour in the upper levels of food chains22,23,24,25. It is also important to note that attacks on other fishes by sharks are not always limited by gape width (despite assertions to the contrary; see ref. 18), because sharks are somewhat unique among larger mesopredators in that they can easily dismember prey (e.g 26.). Thus, the relative sizes of mesopredators will not necessarily be indicative of the response to threats exhibited by smaller species. For example, a recent experimental study by Asunsolo et al.27 animated models of reef sharks and monitored the behaviour of mesopredatory reef fishes when they were confronted by them (Fig. 5). They found that this evoked a reaction of either flight or retreat into shelter by these fishes, behaviours that were most pronounced in the largest species of teleost mesopredator. Based on relative size and gape width, these species should have been the least threatened by the appearance of the predator.
A Asunsolo et al.27 examined the flight initiation distance (FID) of teleost mesopredators (mostly lutjanids, lethrinids and serranids) to the approach of a life-size model of the reef blacktip shark (Carcharhinus melanopterus, a predator and/or lethal competitor), a snorkeler, a model green turtle (Chelonia mydas, a non-threatening object) and object controls (a PVC cylinder and transparent Perspex sheets). Both FID and the speed of flight of teleosts was significantly greater when approached by the model reef shark (and snorkeler) than the green turtle or model controls. Bargraph shows average FID + /− S.E. Points show raw data. B Models and controls were animated on a pulley system and approached from the water column beyond the edge of the reef. Behaviour of the teleost fishes was monitored using a stereo-camera system. C FID was positively correlated with fish size. Plot shows relationship between body length and FID with solid line showing smoothing curve and shaded area standard error of the estimate. Points show raw data. Asunsolo et al.27 suggested a number of (non-exclusive) hypotheses that might account for this relationship, including that larger fish may flee more readily than smaller species as they are older and have more experience with predators, that they may be at greater risk from lethal competition due to their size relative to a reef shark and that they may demonstrate lower risk-taking because they have higher reproductive value (the asset protection principal). Image artwork credit: Dean Tysdale.
It is most likely that any release for CoTS populations from predation by mesopredatory fishes occurs at post-settlement juvenile stages. At settlement, very small benthic juveniles inhabit coral rubble and feed on coralline algae. Once they switch to a diet of coral, they emerge from the rubble and begin a phase of exponential growth in size. These small, post-settlement juveniles are cryptic and nocturnal, suggesting that threat from visual predators is a driver of their behaviours4. They also lack some of the physical defences (long, sharp spines) of adults28. Field studies show that these post-settlement juveniles often have injuries such as missing arms, consistent with encounters with predators28,29,30. Although they are also subject to many predatory invertebrates within the reef (see review by ref. 4), the process of emergence from the rubble is likely to be an important mortality bottleneck4 and one where feeding by mesopredatory fishes could play a significant role.
A reduction in predation pressure, particularly during the early life history of CoTS through the processes described here provides a plausible mechanism for outbreaks of these invertebrates in reef systems. In this situation, the removal of the largest mesopredators by fishing expands the trophic niche of other, smaller mesopredators and reduces the mortality of benthic invertebrates (a trophic cascade; Fig. 6). Given that both top-down and bottom-up processes likely drive outbreaks, a reduction in fishing pressure and a rebuilding of communities of large mesopredators might not necessarily prevent large year classes of larval CoTS settling into reef environments from the plankton but could further constrict the bottleneck of mortality experienced by newly settled juveniles while they reside within the reef matrix or emerge from rubble. Field evidence to support this idea is provided by long term monitoring of coral communities on the GBR12. Over more than a decade on mid-shelf reefs (where the majority of CoTS outbreaks occur), only 20% of reefs that were designated as no-take Marine Protected Areas (MPAs) had outbreaks, whereas outbreaks occurred on 75% of reefs that were open to fishing (Fig. 1B). When reefs at all shelf positions were considered (inner, mid-, and outer shelf), outbreaks occurred on only 8% of protected reefs but afflicted 57% of unprotected reefs. Reefs managed as no-take MPAs remain some of the few places on the GBR where diver surveys show that reef sharks are still abundant31, likely due to constraints on fishing. Similarly, Kroon et al.11 investigated densities of mesopredatory fishes (labrids, lethrinids, lutjanids and serranids) and CoTS on 56 pairs of fished and unfished reefs across the whole of the GBR between 2006 and 2022. Over this time, they found that CoTS were 2.8 times more abundant on fished than unfished reefs (Fig. 1D).
A Prior to fishing, reef sharks are present on reefs and smaller mesopredatory teleosts feed on both benthic invertebrates and some pelagic species. B After fishing, reef sharks are removed, mesopredatory teleosts are lower in abundance and remaining individuals move into the water column, mostly feeding on pelagic prey. Populations of benthic invertebrates, including CoTS, are released from predation pressure and form outbreaks. Image artwork credit: Dean Tysdale.
The largest mesopredatory fishes, including sharks, are typically the first species to be removed by fishing from reef systems. These species occur at naturally low abundances and in the case of sharks are uniquely vulnerable to fishing pressure due to their k-selected life history strategies32. Around the globe, fishing has been so effective at removing sharks that these animals are now functionally extinct on 20% of the world’s coral reefs33. Even the most common, widespread species have suffered major declines in abundance across their ranges34. Although sharks may not be the primary target of fishers, their fins have high market value, and they are frequently caught as by-catch in reef fisheries. As feeding and behavioural studies show, this loss of mesopredators may have had a fundamental impact on reef community structure and the likelihood of outbreaks of CoTS.
How do we test for the presence of a trophic cascade?
Evidence for trophic cascades, particularly in the complex trophodynamics of a coral reef is notoriously contentious19,35 and it is both ethically questionable and logistically difficult to exclude or remove large mesopredators from the substantial areas of reef over which they roam. Yet, opportunities to test the predictions of our hypotheses do exist. For example, recent research shows that populations of large mesopredatory fishes including sharks can recover relatively swiftly if protection from fishing is enforced at whole reef scales36. This could provide the opportunity to experimentally examine changes in populations of benthic invertebrates following the recovery of reef shark populations (Fig. 7). Evidence that the rebuilding of populations of large mesopredators might impact benthic invertebrates is shown by Ningaloo Reef, where outbreaks of the horn drupe gastropod (Drupella spp.) were once a major threat to reefs and were associated with considerable declines in coral cover37. Since 1994, a few years after the designation of the Ningaloo Marine Park in Western Australia in 1987, populations of large mesopredators including sharks have rebounded and the densities of Drupella have declined over time from outbreaks to low or moderate numbers38. As noted above, long-term monitoring programs also provide correlative evidence between outbreak frequencies and fish community structure11,12. Such studies using monitoring data possibly offer the most tractable approach, given that places where increased protection allows fish communities to return to unharvested levels are relatively rare. Importantly, in the case of monitoring the recovery of fish communities, protection from fishing must occur at scales sufficient to encompass the movement patterns of the largest mesopredators, including sharks (i.e., whole reefs or greater in archipelagic systems39). An alternative approach might involve using chemical tags, such as isotopes, in experimental studies to trace the consumption of juvenile CoTS by mesopredators on reefs that differ in the abundance of reef sharks and other larger mesopredators (Fig. 7).
a A field study across a network of paired reefs (no-take and fished) with juvenile CoTS marked with a tracing biomarker (e.g., a stable isotope). After marking, reefs are revisited to measure levels of tracer in smaller mesopredators. If large mesopredators are more abundant on no-take reefs and are forcing smaller mesopredators to predate on invertebrates such as CoTS, one would expect tracer levels in small mesopredators to be higher on no-take reefs than on fished reefs. b An expansion of the first scenario using a Before-After-Control-Impact (BACI) experimental design, where juvenile CoTS are cultured in the laboratory with an elevated level of tracer, then deployed within experimental sites monitored by cameras. Video cameras identify the smaller mesopredatory fishes that consume CoTS and these would be targeted for measurement of tracer. Similar to the first scenario, after deployment of the labeled CoTS, it would be expected that the levels of tracer in these small mesopredators would be higher at no-take reefs than on fished reefs. c A series of reefs along a gradient of abundance of larger mesopredators would be visited, and smaller mesopredators would be collected for DNA analysis of guts. As in the previous scenarios, on reefs where large mesopredators are abundant, we would expect to find higher numbers of reads of CoTS DNA in the gut contents of small mesopredators. The exact functional form of the relationship between abundances of large mesopredators and reads of CoTS DNA is unknown; two possible relationships are shown (dashed and dotted lines). Characterizing this relationship might help quantify the minimum abundances of large mesopredators required to reduce the incidence of CoTS outbreaks. These three scenarios are not mutually exclusive and could be deployed in various combinations.
Future directions
At present we lack a clear understanding of food chains in coral reef ecosystems. We have little knowledge of the role of predation and its impact on the structure and flow of trophic relationships. This is because firstly, we have lacked adequate analytical tools to detect predator-prey relationships among species in feeding studies and secondly, because predation events are extremely difficult to observe directly, particularly for large mesopredators that move over substantial home ranges. Furthermore, the role of predation in modifying population outbreaks of CoTS is most likely occurring at the early life stages, immediately following settlement and/or as recently settled juveniles emerge to feed on coral. Monitoring the survivorship of these small individuals within the matrix of coral rubble they inhabit is a major logistic challenge4. Fortunately, the advent of DNA analysis of gut contents11, animal borne cameras40 and remote underwater camera systems17 now provide the means to gain more insights into predator-prey relationships even within the reef matrix. The use of models to elicit threat responses in prey has also provided a tractable means to assess risk behaviours in an experimental setting. However, a lack of information is compounded by the ubiquity of fishing in coral reef ecosystems. There are now very few reefs that are not subject to some form of fishing pressure and many with no-take Marine Protected Area status are not of a size sufficient to protect the largest mesopredators such as sharks, which have large (km-10’s km) home ranges41. As we show here, evidence provided by new feeding and behavioural studies of predator threat responses in mesopredatory fishes provide plausible mechanisms to suggest that fishing may fundamentally alter trophic relationships in coral reefs systems and ultimately provide opportunities for population release for invertebrates including CoTS. This occurs through both a reduction in direct predation and by altering the landscape of fear experienced by those mesopredators that remain within these habitats (Fig. 6). This does not exclude a contribution by bottom-up processes such as nutrient enrichment in driving year class success in CoTS outbreaks. It does, however, provide an explanation for why outbreaks might occur at isolated reefs remote from terrestrial sources of enrichment. Such reefs may not receive coastal nutrients, but like nearly all other coral reefs, they are subject to various levels of fishing pressure.
If we are to improve the resilience of coral reefs in the face of multiple anthropogenic threats, we require a better understanding of how food chains work in coral reefs in terms of both direct and indirect responses to predation. Our synthesis of feeding and behavioural studies suggests that one of the simplest ways to increase the likelihood of reef survival might be to reduce fishing pressure. When implemented at sufficient scale, this could provide an experimental framework to test the role of predation as a driver of outbreaks and to disentangle the relative effects of top-down and bottom-up processes. Unlike climate change and nutrient inflows from water catchments dominated by agriculture, both of which will take many decades to halt or reverse, populations of mesopredators can rebound in reef systems within a few years once fishing ceases36. Consequently, outbreaks of CoTS might be one the few key causes of the current coral reef crisis that can be mitigated effectively on relatively short time scales.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are reproduced from published work.
References
Hughes, R. N., Hughes, D. J. & Smith, I. P. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp). Oceanogr. Mar. Biol.: Annu. Rev. 52, 133–200 (2014).
Uthicke, S., Pratchett, M. S., Bronstein, O., Alvarado, J. J. & Wörheide, G. The crown-of-thorns seastar species complex: knowledge on the biology and ecology of five corallivorous Acanthaster species. Mar. Biol. 171, 32 (2024).
Pratchett, M. S. et al. Thirty years of research on crown-of-thorns starfish (1986–2016): scientific advances and emerging opportunities. Diversity 9, 41 (2017).
Cowan, Z.-L., Pratchett, M., Messmer, V. & Ling, S. Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 9, 7 (2017).
Fabricius, K., Okaji, K. & De’Ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29, 593–605 (2010).
Brodie, J., Devlin, M. & Lewis, S. Potential enhanced survivorship of crown of thorns starfish larvae due to near-annual nutrient enrichment during secondary outbreaks on the central mid-shelf of the Great Barrier Reef, Australia. Diversity 9, 17 (2017).
Matthews, S., Mellin, C. & Pratchett, M. S. in Advances in Marine Biology Vol. 87 223-258 (Elsevier, 2020).
Mellin, C. et al. Spatial resilience of the Great Barrier Reef under cumulative disturbance impacts. Glob. Change Biol. 25, 2431–2445 (2019).
Babcock, R. C. et al. Suppressing the next crown-of-thorns outbreak on the Great Barrier Reef. Coral Reefs 39, 1233–1244 (2020).
Dulvy, N. K., Freckleton, R. P. & Polunin, N. V. Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol. Lett. 7, 410–416 (2004).
Kroon, F. J., Barneche, D. R. & Emslie, M. J. Fish predators control outbreaks of Crown-of-Thorns Starfish. Nat. Commun. 12, 1–10 (2021).
Sweatman, H. No-take reserves protect coral reefs from predatory starfish. Curr. Biol. 18, R598–R599 (2008).
Pratchett, M. S. et al. Knowledge gaps in the biology, ecology, and management of the Pacific crown-of-thorns sea star Acanthaster sp. on Australia’s Great Barrier Reef. Biol. Bull. 241, 330–346 (2021).
Kroon, F. J. et al. DNA-based identification of predators of the corallivorous Crown-of-Thorns Starfish (Acanthaster cf. solaris) from fish faeces and gut contents. Sci. Rep. 10, 1–14 (2020).
Barley, S. C., Meekan, M. G. & Meeuwig, J. J. Diet and condition of mesopredators on coral reefs in relation to shark abundance. PloS one 12, e0165113 (2017).
Lester, E. K., Langlois, T. J., Simpson, S. D., McCormick, M. I. & Meekan, M. G. The hemisphere of fear: the presence of sharks influences the three dimensional behaviour of large mesopredators in a coral reef ecosystem. Oikos 129, 731–739 (2020).
Lester, E., Langlois, T., Simpson, S., McCormick, M. & Meekan, M. Reef-wide evidence that the presence of sharks modifies behaviors of teleost mesopredators. Ecosphere 12, e03301 (2021).
Barley, S. C., Clark, T. D. & Meeuwig, J. J. Ecological redundancy between coral reef sharks and predatory teleosts. Rev. Fish. Biol. Fish. 30, 153–172 (2020).
Roff, G. et al. The ecological role of sharks on coral reefs. Trends Ecol. Evol. 31, 395–407 (2016).
Robbins, W. & Renaud, P. Foraging mode of the grey reef shark, Carcharhinus amblyrhynchos, under two different scenarios. Coral Reefs 35, 253–260 (2016).
Lester, E. K. et al. Relative influence of predators, competitors and seascape heterogeneity on behaviour and abundance of coral reef mesopredators. Oikos 130, 2239–2249 (2021).
Gordon, C. E., Feit, A., Grüber, J. & Letnic, M. Mesopredator suppression by an apex predator alleviates the risk of predation perceived by small prey. Proc. R. Soc. B: Biol. Sci. 282, 20142870 (2015).
Ritchie, E. G. & Johnson, C. N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998 (2009).
Sivy, K. J., Pozzanghera, C. B., Grace, J. B. & Prugh, L. R. Fatal attraction? Intraguild facilitation and suppression among predators. Am. Nat. 190, 663–679 (2017).
Merkle, J., Stahler, D. R. & Smith, D. W. Interference competition between gray wolves and coyotes in Yellowstone National Park. Can. J. Zool. 87, 56–63 (2009).
Mourier, J. et al. Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Curr. Biol. 26, 2011–2016 (2016).
Asunsolo-Rivera, A. et al. Behaviour of mesopredatory coral reef fishes in response to threats from sharks and humans. Sci. Rep. 13, 6714 (2023).
Wilmes, J. C., Hoey, A. S., Messmer, V. & Pratchett, M. S. Incidence and severity of injuries among juvenile crown-of-thorns starfish on Australia’s Great Barrier Reef. Coral Reefs 38, 1187–1195 (2019).
Messmer, V., Pratchett, M. & Chong-Seng, K. Variation in incidence and severity of injuries among crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 9, 12 (2017).
Rivera-Posada, J., Caballes, C. F. & Pratchett, M. S. Size-related variation in arm damage frequency in the crown-of-thorns sea star, Acanthaster planci. J. Coast. Life Med. 2, 187–195 (2014).
Robbins, W. D., Hisano, M., Connolly, S. R. & Choat, J. H. Ongoing collapse of coral-reef shark populations. Curr. Biol. 16, 2314–2319 (2006).
Cortés, E. Life history patterns, demography, and population dynamics. Biol. Sharks Relatives. 1, 449–469 (2004).
MacNeil, M. A. et al. Global status and conservation potential of reef sharks. Nature 583, 801–806 (2020).
Simpfendorfer, C. A. et al. Widespread diversity deficits of coral reef sharks and rays. Science 380, 1155–1160 (2023).
Ruppert, J. L., Fortin, M.-J. & Meekan, M. G. The ecological role of sharks on coral reefs: Response to Roff et al. Trends Ecol. Evol. 31, 586–587 (2016).
Speed, C. W., Cappo, M. & Meekan, M. G. Evidence for rapid recovery of shark populations within a coral reef marine protected area. Biol. Conserv. 220, 308–319 (2018).
Turner, S. J. The biology and population outbreaks of the corallivorous gastropod Drupella on Indo-Pacific reefs. Oceanogr. Marine Biol. Annu. Rev. 32, 461–530 (1994).
Holmes, T. et al. Ecological monitoring in the Ningaloo marine reserves 2017. Department of Biodiversity, Conservation and Attractions, Perth (2017).
Bonnin, L. et al. Recent expansion of marine protected areas matches with home range of grey reef sharks. Sci. Rep. 11, 1–11 (2021).
P. O’Connell, C., Collatos, C., Picha, N. D. & He, P. A new animal-borne imaging system for studying the behavioral ecology of small sharks: laboratory and field evaluations. Mar. Freshw. Behav. Physiol. 53, 131–150 (2020).
Dwyer, R. G. et al. Individual and population benefits of marine reserves for reef sharks. Curr. Biol. 30, 480–489.e485 (2020).
Langlois, T. et al. A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods Ecol. Evol. 11, 1401–1409 (2020).
Acknowledgements
This work was supported by the University of Western Australia and the Australian Institute of Marine Science.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.G.M., D.B. Visualization: M.G.M. Writing—original draft: M.G.M., D.B. Writing—review & editing: M.G.M, D.B., E.L., F.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Morgan Pratchett and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Tobias Goris. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meekan, M.G., Lester, E.K., Kroon, F.J. et al. Predator removals, trophic cascades and outbreaks of crown-of-thorns starfish on coral reefs. Commun Biol 8, 305 (2025). https://doi.org/10.1038/s42003-025-07716-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07716-6