Abstract
The chaperonin tailless complex polypeptide 1 (TCP1) is a key subunit of chaperonin containing TCP1 (CCT) that regulates the folding and stability of proteins during cancer progression. Here, the prognostic significance of TCP1 was explored mainly in patients with hepatocellular carcinoma (HCC) and pancreatic ductal adenocarcinoma (PDAC). We showed that TCP1 expression was significantly greater in clinically malignant tumour tissues than in normal tissues and that high TCP1 expression was associated with poor prognosis. TCP1 suppression not only decreased the proliferation and invasion of cancer cells in vitro but also inhibited tumour growth and metastasis in vivo. The underlying mechanisms were determined by ubiquitination assays and Co-IP (Co-Immunoprecipitation) experiments, and it was found that TCP1 regulated the stability of c-Myc through the RAC-alpha serine/threonine-protein kinase (AKT) /Glycogen synthase kinase 3β (GSK-3β) and extracellular regulated protein kinases (ERK) signalling pathways. Moreover, TCP1 knock-in (TCP1-KI) dramatically promoted the occurrence of diethylnitrosamine (DEN) -induced primary HCC in mice. Our results highlight the critical role of TCP1 in HCC and PDAC and reveal a novel mechanism to suppress HCC and PDAC by targeting c-Myc via the TCP1-induced promotion of the AKT/GSK-3β and ERK signalling pathways. TCP1 is able to modulate the stability of target proteins by multiple pathways, thus promoting the progression of HCC and PDAC. Our study identifies TCP1 as a prognostic novel marker and therapeutic target of HCC and PDAC.
Similar content being viewed by others
Introduction
Molecular chaperone CCT (chaperonin containing TCP1) is the main component involved in maintaining the proteostasis network. It plays important roles in protein biosynthesis, folding, translocation, assembly, degradation, and protein homoeostasis through a complex cooperative mechanism1,2. It has been reported that CCT assists in the folding and assembly of ~10–15% of cytoplasmic neoproteins3. CCT contains eight subunits (CCT1–CCT8), which form a chaperone ring structure4,5. Previous studies have shown that the high expression of CCT subunits is closely related to the occurrence and development of cancers6,7,8,9,10,11,12. Importantly, increasing evidence indicates that CCT mediates the development and progression of cancer by interacting with oncogenic factors that regulate tissue growth and apoptosis13,14,15,16,17, indicating that CCT may participate in the process of cancer as a regulatory factor of proto-oncogenes. At present, most of the research on CCT focuses on the entire CCT complex or the subunits CCT3 and CCT6, and little is known about the specific mechanism of the first subunit of CCT, TCP118,19. Our previous studies have indicated that TCP1, a subunit of CCT (CCT1), may be a potential biomarker for hepatocellular carcinoma (HCC), and we demonstrated that TCP1 regulates the Wnt7b/β-catenin pathway through P53 to influence the proliferation and migration of hepatocellular carcinoma cells. Compared to patients with low TCP1 expression, patients with high TCP1 expression had shorter overall survival (OS) or disease-free survival (DFS) and a greater hazard ratio (HR) in HCC20. Our recent studies find that high TCP1 expression enhances the sensitivity of lymphoma tumour cell to ferroptosis21. However, little is known about the function of TCP1 in the pathogenesis of cancers other than HCC and pancreatic ductal adenocarcinoma (PDAC).
As mentioned previously, TCP1 can not only assist in the folding of proteins as a chaperone but also regulate the anti-oncogene p53. Thus, we speculate that TCP1 may also regulate other cancer-related genes (including oncogenes and anti-oncogenes) during tumour progression. The stabilization and degradation of oncogenic proteins are essential for tumour formation, development, and metastasis22. As a multifunctional transcription factor, the oncoprotein c-Myc is hyperactivated in most human cancers and is closely associated with poor clinical patient prognosis23,24. The stabilization of c-Myc is related to tumour initiation and tumour progression in various cancers, including HCC and PDAC24,25. The stability and activity of the c-Myc protein are strictly regulated by its phosphorylation-dependent degradation via the ubiquitin‒proteasome system. The phosphorylation of threonine 58 (T58) and serine 62 (S62) of the c-Myc protein is involved in regulating its stability26. A variety of signalling pathways have been shown to modulate the phosphorylation of c-Myc, thereby affecting its protein stability and function in cells. For example, the PI3K/AKT pathway, which is commonly mutated in cancers27, can modulate c-Myc protein stability via a series of transduction cascades28,29,30. GSK-3β is the target of PI3K/AKT-mediated phosphorylation at Ser9. AKT, as an effector kinase, phosphorylates the Ser9 site of GSK-3β and causes its loss of kinase activity27; AKT can phosphorylate c-Myc at residue T58 and subsequently ubiquitinate the c-Myc protein. In addition, the S62 residue of c-Myc can be phosphorylated by extracellular regulated protein kinases (ERKs) or cyclin-dependent kinases (CDKs), stabilizing the c-Myc protein in cells31,32. The stabilization of c-Myc increases its transcriptional activity, promoting the expression of downstream target genes33. However, whether and how TCP1 regulates the ability of c-Myc to participate in cancer progression remain to be investigated.
In this study, we identified TCP1 as a novel biological marker of HCC and PDAC. Here, we showed that TCP1 was prevalently overexpressed in multiple human solid cancers and that high TCP1 expression indicated shorter survival in HCC and PDAC patients. Genetic TCP1 inhibition significantly decreased the proliferation, migration, and invasion of HCC and PDAC cells in vitro and in vivo by regulating the stability of c-Myc via the AKT/GSK-3β and ERK signalling pathways, respectively. Furthermore, TCP1 potently promoted primary HCC induced by diethylnitrosamine (DEN) in TCP1-KI mice. Taken together, our findings demonstrated the role of TCP1 in HCC and PDAC. TCP1 might function as a tumour-promoting factor, play a crucial role in the tumorigenesis process, and serve as a promising therapeutic target for cancers.
Results
TCP1 is highly expressed in many solid tumours and is related to poor outcomes in patients with HCC and PDAC
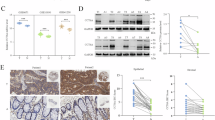
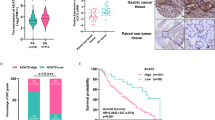
To investigate the generalizability of high TCP1 expression levels in human solid tumours, we first examined TCP1 expression in tumour and normal tissues using datasets from The Cancer Genome Atlas (TCGA) database https://portal.gdc.cancer.gov/) and The Genotype-Tissue Expression (GTEx) database (https://www.gtexportal.org/). We then performed survival analysis on data from TCGA. We found that the levels of TCP1 expression were greater in most solid tumour tissues, including breast cancer, lung cancer, colorectal cancer, gastric cancer, pancreatic cancer, head and neck cancer, and liver cancer tissues than in normal groups (Fig. 1a). Kaplan–Meier survival analysis revealed that high TCP1 expression was correlated with significantly shorter overall survival (OS) in patients with breast cancer (p < 0.0001) and lung cancer (p = 0.017) (Fig. 1b). We further verified the expression of TCP1 in various cancers, including HCC, PDAC, lung cancer, lymphoma, gastric cancer, and thyroid cancer. The average level of TCP1 in tumour tissues was greater than that in adjacent tissues in all types of cancer examined (Fig. 1c), indicating that TCP1 is markedly increased in many solid tumours. Subsequently, we focused on the expression of TCP1 in liver and pancreatic cancers by IHC (immunohistochemistry) and IHF (Immunohistofluorescence). The results showed that the expression levels of TCP1 in poorly differentiated HCC and PDAC were greater than those in well-differentiated and moderately differentiated tumours (Fig. 1d, f, h), and Pearson correlation analysis revealed that the TCP1 level was negatively correlated with recurrence time in patients with HCC and PDAC (Fig. 1e, g). Furthermore, survival analysis indicated that the overall survival (OS) and disease-free survival (DFS) of patients with high levels of TCP1 expression were significantly shorter than those of patients with low levels of TCP1 in PDAC, suggesting that TCP1 is associated with poor prognosis in patients with malignant tumours (Fig. 1i, j).
a The levels of TCP1 expression were greater in most solid tumour tissues than in normal groups in TCGA database. ACC adrenocortical cancer, BLCA bladder cancer, BRCA breast cancer, CESC cervical cancer, CHOL bile duct cancer, COAD colon adenocarcinoma, DLBC large B-cell lymphoma, ESCA esophageal cancer, GBM glioblastoma, HNSC head and neck cancer; KICH kidney chromophobe, KIRC kidney chromophobe, KIRP kidney chromophobe, LAML acute myeloid leukaemia, LGG acute myeloid leukaemia, LIHC liver cancer, LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, OV ovarian cancer, PAAD pancreatic cancer, PCPG pheochromocytoma & paraganglioma, PRAD prostate cancer, READ prostate cancer, SARC sarcoma, SKCM melanoma, STAD stomach cancer, TGCT testicular cancer, THCA thyroid cancer, THYM thymoma, UCEC endometrioid cancer, UCS uterine carcinosarcoma. b Kaplan–Meier survival analysis revealed that high TCP1 expression was correlated with significantly shorter overall survival (OS) in patients with breast cancer and lung cancer. c IHC was carried out, and the relative TCP1 staining intensity was scored to analyse TCP1 expression levels in adjacent and malignant tumours. d, f, h Fluorescence imaging and IHC were used to determine the relationship between TCP1 expression and the degree of differentiation in liver and pancreatic cancers. Representative images and the relative scores of TCP1 are shown for liver cancer d and pancreatic cancer f, h tissues. Scale bar, 200 μm. e, g Pearson correlation analysis revealed that the TCP1 level was negatively correlated with recurrence time in patients with HCC (e) and PDAC (g). i, j Relationships between TCP1 expression and overall survival (OS) (i) and disease-free survival (DFS) (j) rates in pancreatic cancer patients. The data are shown as the means ± SDs from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, no significance, between the indicated groups.
Suppression of TCP1 inhibits the proliferation, migration, and invasion of HCC and PDAC cells
To investigate the effect of TCP1 on the phenotypes of HCC and PDAC cells, we conducted functional experiments to evaluate the influence of TCP1 knockdown on cell proliferation, migration, and invasion. First, Huh-7, SMMC-7721, PANC-1, and BxPC-3 cells were infected with lentivirus containing shRNA targeting TCP1. TCP1 protein expression was significantly decreased in all shTCP1 cell lines (Fig. 2a). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and colony formation assays showed that shTCP1 significantly inhibited cell growth and colony formation (Fig. 2b, c). Next, we examined the effect of shTCP1 on cell migration by Transwell assays. shTCP1 significantly inhibited the migration and invasion of the four cell lines (Fig. 2d). Taken together, TCP1 knockdown successfully attenuated the proliferation, migration, and invasion of various cancer HCC and PDAC cell models in vitro.
a A lentiviral system was used to induce stable expression of shRNA targeting TCP1, and TCP1 expression was detected by WB. b, c MTT (b) and colony formation (c) assays were used to assess the growth of Huh-7, SMMC-7721, PANC-1, and BxPC-3 cells. d Transwell assays were carried out to analyse the migration and invasion abilities of cells. The data are shown as the means ± SD from three independent experiments. **P < 0.01, ***P < 0.001, between the indicated groups.
Suppression of TCP1 expression inhibits tumour growth and metastasis in mouse models
To determine the effect of altered TCP1 expression on tumour proliferation, we established a xenograft model in nude mice using the Huh-7, SMMC-7721, and PANC-1 cancer cell lines. Consistent with the in vitro results, xenograft tumours with downregulated TCP1 expression grew more slowly than control xenografts (Fig. 3a). Compared with the controls, TCP1 suppression significantly inhibited growth as tumour volume decreased and tumour weight decreased in all three cell lines (Fig. 3b, c). To further investigate the role of TCP1 in HCC and PDAC metastasis, we injected SMMC-7721 and BxPC-3 cells labelled with luciferase into the tail veins of mice to track metastasis. The metastatic capacities of liver cancer and pancreatic cancer cells in vivo were then detected by a live imaging system. Moreover, knocking down TCP1 significantly reduced metastasis in both SMMC-7721 and BxPC-3 cells (Fig. 3d, e). Furthermore, the OS of mice treated with shTCP1 cancer cells was significantly greater than that of control mice (Fig. 3f). These results suggest that the knockdown of TCP1 could significantly decrease the tumour burden in vivo, thus increasing the survival rate of mice.
Xenograft tumour and tail vein transport models were constructed with stable expression of shRNA targeting TCP1 (shTCP1). a–c Xenograft tumour models were constructed with SMMC-7721 (n = 12), Huh7 (n = 10) and PANC-1 (n = 10) cells. a Photographs of tumours collected from nude mice. b TCP1 knockdown modulated tumour volume growth in vivo. c TCP1 knockdown induced tumour weight loss in vivo (measured at the endpoint). d–f A live imaging system for small animals was used to assay the metastatic ability of HCC and PDAC cell lines in vivo. Tail vein transport models were constructed with SMMC-7721 and BxPC-3-luciferase cells harbouring TCP1 shRNA or control vector (n = 10 per group). d Photographs of tissues collected from nude mice. e The intensity of the contrast band indicated that TCP1 knockdown decreased metastasis in vivo. f Kaplan–Meier survival analysis of knockdown and control mice. The data are shown as the mean ± SD. One-way ANOVA and Student’s t-test were used; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, no significance.
Knockdown of TCP1 accelerates the degradation of c-Myc via the ubiquitin‒proteasome system
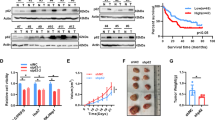
Our in vitro and in vivo results showed that TCP1 had a strong effect on tumour cell proliferation. To better understand the molecular mechanism of TCP1-mediated tumour progression, we examined whether TCP1 inhibition affects the core signalling networks that are known to play important roles in HCC and PDAC, including the PI3K/AKT/mTOR and MAPK signalling pathways and the key genes involved in cell proliferation. According to the WB results (Supplementary Fig. 1), we assessed the influence of TCP1 on the expression of the tumour proliferation-related protein c-Myc in TCP1-knockdown cell models. The results showed that the protein level of c-Myc, but not the mRNA level, was significantly decreased (Fig. 4a, b). Therefore, we speculated that TCP1 could affect the stability of the c-Myc protein. Subsequently, we used the protein synthesis inhibitor cycloheximide (CHX) to measure the degradation of the c-Myc protein. We found that the degradation of c-Myc was faster in cancer cells with TCP1 knockdown than in control cells, suggesting that TCP1 may regulate the protein stability of c-Myc (Fig. 4c). Then, MG132 was added to inhibit the activity of the 26S proteasome. The degradation of the c-Myc protein was significantly inhibited in cancer cell models treated with both CHX and MG132 compared with those treated with CHX/ alone (Fig. 4c). Moreover, we observed that the degradation rate of c-Myc was considerably greater in the shTCP1 group than in the scramble group for all types of cancer examined (Fig. 4d. Subsequently, using ubiquitination assays, we found that TCP1 knockdown increased the level of ubiquitinated c-Myc (Fig. 4e). The above results indicated that suppression of TCP1 expression promotes the ubiquitination of c-Myc in cells, eventually accelerating the degradation of the c-Myc protein.
a, b TCP1 was knocked down, and the expression levels of c-Myc and TCP1 in Huh-7, SMMC-7721, PANC-1, and BxPC-3 cells were detected by WB (a) and RT‒qPCR (b). c, d Cells were treated with 50 μM CHX and 10 μM MG132 or DMSO. The protein levels of TCP1 and c-Myc were determined by WB (c). Huh-7 cells were treated with CHX for 0, 25, 35, or 50 min. SMMC-7721 cells were treated with CHX for 0, 10, 20, or 30 min. PANC-1 cells and BxPC-3 cells were treated with CHX for 0, 15, 25, or 40 min. Then, the protein levels of TCP1 and c-Myc were determined by WB, and the relationship between CHX chase time and the relative c-Myc level was analysed (d). e Ubiquitination assays were carried out to observe the ubiquitination level of c-Myc in the shTCP1+Flag-c-Myc+HA-ubiquitin+MG132 and scramble+Flag-c-Myc+HA-ubiquitin+MG132 groups. The data are shown as the means ± SDs from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significance, between the indicated groups.
TCP1 regulates the stability of c-Myc through the AKT/GSK-3β and ERK signalling pathways
Previous studies have shown that the phosphorylation of T58 decreases the stability of c-Myc and promotes its degradation via the ubiquitin‒proteasome system, conversely, the phosphorylation of S62 stabilizes c-Myc31,32. It has been reported that the phosphorylation of c-Myc is regulated mainly by the AKT/GSK-3β and ERK pathways26. Therefore, we sought to determine whether TCP1 regulates c-Myc protein stability through its phosphorylation. We manipulated the TCP1 expression levels in the above four cell lines and determined changes in the phosphorylation of c-Myc. While the total protein level of c-Myc and the ratio of p-c-Myc S62 to p-c-Myc T58 was decreased in TCP1 knockdown cells, overexpression of TCP1 had the opposite effect, providing evidence that TCP1 regulates the stability of c-Myc mainly by altering the ratio of p-c-MycS62 to p-c-MycT58 (Fig. 5a–c). Then, we assessed the levels of key proteins of the AKT/GSK-3β and ERK signalling pathways. The phosphorylation levels of AKTS473, GSK-3βS9, and ERK, especially the p-AKTS473/AKT, p-GSK-3βS9/GSK-3β, and p-ERK/ERK ratios, were significantly reduced with TCP1 knockdown but increased with TCP1 overexpression (Fig. 5a–c). These results suggest that TCP1 can regulate the phosphorylation of key proteins in the AKT/GSK-3β and ERK signalling pathways to determine the stability or degradation of the c-Myc protein. To further validate the role of TCP1 in the regulation of c-Myc, we explored whether the change in c-Myc expression is directly associated with TCP1 expression. Against S9, Y216 is an active phosphorylation site of GSK-3β. With AR-A014418 (AR) treatment to inhibit GSK-3β activity, we found that the protein levels of c-Myc increased in all four cell lines (Fig. 5d, h) and these trends did not change after TCP1 ablation (Fig. 5e, g, i). Conversely, we performed a comparative study to detect the TCP1 protein levels following inhibition of GSK-3β. After treatment with AR-A014418, the inhibitor of GSK-3β at different concentrations in HCC cells and PDAC cells, we found the ratio of p-GSK-3βY216/ GSK-3β has decreased significantly (Supplementary Fig. 2a, b), respectively, whereas the TCP1 protein levels do not change simultaneously (Supplementary Fig. 2a, c). Next, we used different concentrations of the AKT-specific inhibitor, AKT inhibitor VIII (AKT-iVIII), to inhibit AKT activity in the four cell lines. The results showed that the protein levels of c-Myc decreased irrespective of the TCP1 level (Fig. 5f, j), indicating that the TCP1 protein is an upstream regulator of the AKT/GSK-3β pathway. These results revealed that TCP1 regulates the stability of the c-Myc protein through the AKT/GSK-3β and ERK signalling pathways, suggesting that TCP1 may play a critical role in disrupting proteostasis by driving the oncogene c-Myc.
a–c TCP1 was knocked down, and the levels of c-Myc, p-c-MycT58, p-c-MycS62, TCP1, AKT, p-AKTS473, GSK-3β, and p-GSK-3βS9 in Huh-7 and SMMC-7721 cells were detected by WB (a). The same detection markers were assessed in PANC-1 and BxPC-3 cells (b). The phosphorylation levels of AKTS473, GSK-3βS9, and ERK, especially the p-AKTS473/AKT, p-GSK-3βS9/GSK-3β and p-ERK/ERK ratios, were significantly reduced with TCP1 knockdown but increased with TCP1 overexpression (c). d, h The GSK-3β inhibitor AR-A014418 was used to confirm the effect of TCP1 on the AKT/GSK-3β axis. Huh-7 cells were treated with 200 nM and 15 μM AR-A014418, and SMMC-7721, PANC-1, and BxPC-3 cells were treated with 200 nM and 5 μM AR-A014418; then, the levels of p-GSK-3β Y216 and c-Myc were detected. e, g, i AR-A014418 was used in scramble and shTCP1 cells to measure c-Myc protein levels. f, j AKT inhibitor VIII (AKT iVIII) was used to determine the relationship between TCP1 and the AKT/GSK-3β pathway. Huh-7 cells were treated with 1 and 10 μM AKT iVIII, SMMC-7721 cells were treated with 1 and 5 μM AKT iVIII, and PANC-1 and BxPC-3 cells were treated with 5 and 10 μM AKT iVIII, and the expression levels of TCP1, p-AKTS473 and c-Myc were detected. The data are shown as the means ± SD from three independent experiments. *P < 0.05, **P < 0.01, between the indicated groups.
Knocking in TCP1 in a DEN-induced mouse model promotes the expression of c-Myc by regulating the AKT/GSK-3β and ERK signalling pathways, thus facilitating the occurrence of primary liver cancer
Treatment with DEN can induce hepatocarcinogenesis, which is a common feature of human HCC. To gain global insight into the mechanism of TCP1 in hepatocarcinogenesis, we used WT and TCP1-KI mice, a genetic mouse that effectively overexpresses TCP1, to construct a primary HCC model induced by DEN (Fig. 6a). In the DEN model mouse cycle, after 48 months, we collected 7 WT mice and 4 TCP1-KI mice. After the mice were euthanized, a total of 17 tumour nodules with volumes ranging from 0.4 to 458.7 mm3 were obtained from the liver tissue of the 7 WT mice, and a total of 29 tumour nodules with volumes ranging from 0.4 to 3959.8 mm3 were detected in the liver tissues of 4 TCP1-KI mice. The number of tumour nodules (red arrows), the liver weight ratio, and the tumour volume were significantly greater in the TCP1-KI group than in the WT group (Fig. 6b, c), and the relationship between the number of tumour nodules and tumour volume is shown in Fig. 6d, indicating that TCP1 promotes the occurrence and development of primary HCC. In contrast with those in the WT group, histological analysis revealed that the liver tissues of the tumours in the TCP1-KI group exhibited a loss of normal hepatic architecture, more severe cytological atypia, and more obvious vacuoles in the cytoplasm and were positive for AFP protein expression (Fig. 6e). In addition, IHC revealed that the expression levels of c-Myc and Ki67 were significantly increased in the TCP1-KI group (Fig. 6g). Furthermore, the influence of TCP1 overexpression on the AKT/GSK-3β and ERK pathways was determined by WB. Similar to the in vitro results, the overexpression of TCP1 activated the AKT/GSK-3β and ERK pathways to promote the expression of c-Myc (Fig. 6f, h), as indicated by increases in the p-AKTS473/AKT, p-GSK-3βS9/GSK-3β and p-ERK/ERK ratios, which further confirmed our in vivo findings.
a Establishment scheme for wild-type and TCP1-ROSA26 knock-in mice and diagram of the DEN-induced mouse primary HCC model. b, c The numbers of tumour nodules (red arrows), the liver weight ratios, and tumour volumes of the WT and TCP1-KI mice were calculated and analysed. Images of hepatomas (b) the liver/body weight ratio, the tumour number, and the tumour volume are shown (c). d The relationship between the number of tumour nodules and tumour volume. e, g HE staining and IHC were carried out to observe the structure of liver tissues and assay the levels of AFP, TCP1, c-Myc, and Ki67 in the WT and TCP1-KI mouse models. Scale bar, 200 μm. f, h c-Myc, p-c-MycT58, p-c-MycS62, AKT, p-AKTS473, GSK-3β, p-GSK-3βS9, p-ERK, and ERK were detected in WT and TCP1-KI mouse tissues to determine the effect of overexpressing TCP1 in vivo. The data are shown as the means ± SD from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, between the indicated groups.
Discussion
Growing evidence has demonstrated that the molecular chaperone CCT is a critical mediator of tumorigenesis15,34,35,36,37,38,39,40. The effects of its subunits, especially TCP1, on cancer have also been studied. Recent studies have demonstrated that TCP1 is essential for the survival of breast cancer cells9. Upregulated TCP1 is correlated with poor prognosis in patients with ovarian cancer41. In addition, TCP1 increases drug resistance in acute myeloid leukaemia42. Based on these results, we speculate that TCP1 is an oncogene that is related to a variety of cancers. Thus, we focused on TCP1 to further explore how this subunit of CCT regulates tumour progression. In this study, we reported that TCP1 was markedly elevated in malignant tumours such as HCC, PDAC, lung cancer, lymphoma, gastric cancer, and thyroid gland cancer. TCP1 was identified as a poor prognostic indicator for both time to recurrence and OS in HCC and PDAC patients in this and our previous studies20, and the results further support the function of TCP1 as an oncogene in cancers.
Cancer is characterized by uncontrolled proliferation and metastasis43,44. Thus, inhibiting the proliferation and metastasis of tumour cells is a crucial therapeutic strategy. Here, functional experiments demonstrated that knockdown of TCP1 dramatically inhibited the growth and metastasis of tumours in vitro and in vivo. The results revealed the functions of TCP1 in tumour cells and highlighted its role in cancers. Given these findings, we considered that TCP1 might contribute to the tumorigenesis and progression of cancers as an oncogene.
Although TCP1 is associated with cancer progression, previous studies have mostly focused on the relationship between the TCP1 and PI3K/AKT pathways and the mechanism by which TCP1 promotes the development of tumours. As an oncogenic transcription factor, c-Myc regulates various biological activities, including proliferation, apoptosis, and carcinogenesis45,46,47,48,49. Accumulating evidence has confirmed that c-Myc can be activated by many mechanisms in cancer cells, including transcriptional regulation, mRNA stabilization, and protein overexpression and stabilization50. Thus, treatments targeting c-Myc may hinder the rapid proliferation of cancer cells51,52. However, therapeutics directly targeting c-Myc are not yet available. We focused on the stability of c-Myc in our studies considering that the high rate of its degradation could acutely regulate its activity53. Our results showed that the protein level of c-Myc was notably reduced after TCP1 knockdown without affecting the mRNA level, illustrating the role of TCP1 in c-Myc protein stability. For the first time, we demonstrated the relationship between TCP1 and c-Myc, which provides a theoretical basis for the study of the molecular mechanism of TCP1.
In this context, our results further demonstrated that the degradation rates of c-Myc in liver and pancreatic cancer cells were dramatically increased after knocking down TCP1 and treating cells with CHX. In addition, the expression of the c-Myc protein could be rescued by MG132. These results further demonstrated that TCP1 could regulate the degradation of the c-Myc protein via the proteasome pathway. Ubiquitination assays revealed that knocking down TCP1 not only affects the degradation rate of c-Myc but also promotes the ubiquitination of c-Myc in cells. Considering the essential functions of c-Myc in tumorigenesis, it is not surprising that the degradation of c-Myc could ultimately result in the suppression of cancers. These results suggested that c-Myc is a link between TCP1 and tumorigenesis. TCP1 can modulate the progression of cancers by regulating the stability of c-Myc, which offers vital insights into the pathogenic mechanism of cancers.
The findings were further supported by analysis of the phosphorylation sites. The stability and activity of the c-Myc protein are strictly regulated by its two conserved phosphorylation sites, T58 and S62. ERK phosphorylates c-Myc at the S62 residue and increases its protein stability. Conversely, GSK-3β phosphorylates c-Myc at the T58 residue and promotes its degradation31. In addition, GSK-3β is regulated by PI3K/AKT through S9 phosphorylation. The phosphorylation of GSK-3β inhibits its ability to phosphorylate c-Myc at T58, thereby stabilizing c-Myc26,54,55. The AKT/GSK-3β and ERK pathways play critical roles in cancer proliferation, invasion, and drug sensitivity56,57,58. We showed that AKT/GSK-3β, p-AKT, p-GSK-3β, c-Myc and the ratio of p-c-MycS62 to p-c-MycT58 were decreased when TCP1 was knocked down in liver and pancreatic cancer cells. The phosphorylation of ERK in the ERK pathway was also decreased. These effects were reversed by TCP1 overexpression in cancer cells. These results revealed that TCP1 regulates the stability of the c-Myc protein through the AKT/GSK-3β and ERK signalling pathways and, for the first time, highlighted the effect of changes in the ratio of p-c-MycS62 to p-c-MycT58, which indicates a unique mechanism of TCP1.
More importantly, to further elucidate the mechanism of TCP1 in HCC, TCP1-KI genetically engineered mice were used to construct a primary HCC model induced by DEN, as DEN-induced mouse hepatocarcinogenesis is characterized by the development of human HCC, such as inflammation and fibrogenesis. Knocking in TCP1 in mice significantly induced the occurrence and development of primary HCC. These results were consistent with those in vitro, which further confirmed the mechanism of TCP1 in cancers and provided a novel theoretical basis for research on TCP1 and cancer treatment. Overall, our identification of TCP1 could serve as a starting point for exploring its functions, increasing our understanding of its molecular chaperones and facilitating the development of novel therapeutic strategies for treating tumours.
There are still some limitations in our current study. First, although we found that the TCP1 expression levels were increased in most solid tumour tissues in public databases, supporting its potential application as a broad-spectrum tumour marker, this finding should be verified in further studies, especially in terms of its relationship with prognosis. Second, although in vivo, in vitro, and clinical sample studies have confirmed that TCP1 is a potential prognostic factor for HCC and PDAC in clinical practice, further validation should be performed in a larger independent cohort or in a multicentre study. An increasing number of valuable results could be accounted for because of the poor prognostic outcomes of patients with HCC and PDAC. Third, although we found that TCP1 may play a critical role in disrupting proteostasis by driving the oncogene c-Myc through the AKT/GSK-3β and ERK signalling pathways in HCC and PDAC, further in-depth studies on the direct interaction between TCP1 and AKT or ERK and the role of TCP1/c-Myc in other cancers are warranted.
In summary, our findings suggest that TCP1 may be a novel target for treating HCC and PDAC. Our data demonstrated that the expression of TCP1 in liver cancer and pancreatic cancer patients was closely correlated with poor patient prognosis. Knocking down TCP1 could regulate the phosphorylation of c-Myc at T58 and S62 and further inhibit the malignant phenotypes of liver cancer and pancreatic cancer cells via the AKT/GSK-3β and ERK signalling pathways. Genetically engineered mice were then utilized to verify the results. For the first time, we confirmed the effect of TCP1 on the AKT/GSK-3β and ERK axes. This study showed that TCP1 is linked to HCC and PDAC via the stabilization of c-Myc. Overall, this study not only describes a new function of TCP1 in cancer-related genes but also provides a unique strategy for tumour treatment through the inactivation of TCP1 to facilitate c-Myc ubiquitination, which suggests that TCP1 acts not only as a member of the type II chaperone CCT but also as a tumour susceptibility factor in HCC and PDAC development.
Methods
Cell lines
The human hepatoma cell lines (SMMC-7721 and Huh-7) and the human pancreatic carcinoma cell lines (PANC-1 and BxPC-3) were purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China). BxPC-3 and SMMC-7721 cells were cultured in RPMI 1640 medium. PANC-1 and Huh-7 cells were maintained in DMEM. All cells were cultured in complete medium supplemented with 10% foetal bovine serum (FBS; HyClone, Logan, Utah, USA) and 1% penicillin/streptomycin (HyClone, Logan, Utah, USA) at 37 °C in a humidified incubator with 5% CO2.
Animals
Six- to eight-week-old BALB/c nu/nu mice purchased from Shanghai Laboratory Animal Co., Ltd. (SLAC, Shanghai, China) were raised under specific pathogen-free conditions. BALB/c nude mice were housed in laminar flow cabinets with free access to food and water at the Laboratory Animal Centre of Fujian Medical University (FJMU). Ethical approval for animals: All animal experiments were performed following the animal protocols and regulations approved by the Animal Ethics Committee of FJMU (IACUC-FJMU 2022-Y-0541). We have complied with all relevant ethical regulations for animal use.
Patients and specimens
All solid tumour samples, including liver cancer, pancreatic cancer, lung cancer, lymphoma, gastric cancer and thyroid carcinoma samples, were collected from the FJMU Union Hospital or First Affiliated Hospital of FJMU between 2015 and 2017. No patient received chemotherapy or radiotherapy before the operation. Overall survival (OS) was defined as the duration from the date of initial diagnosis to the date of death. None of the patients died within 1 month after surgery. Tissue slides were cut into 4-µm sections for immunochemistry (IHC) and immunohistofluorescence (IHF), and all sections were examined and scored independently by two investigators in a double-blinded manner. The staining intensity was determined according to a histological scoring method. We quantified the staining intensity and percentage of stained cells, and positive tumour cells were quantified by two independent pathologists. Staining intensity was scored from 0 to 3: negative (0), weak (1), medium (2), and strong (3). The degree of staining (the percentage of the positive staining area of tumour cells in the whole tumour area) was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) or 4 (76–100%). The total protein expression score (overall score range, 0–12) was calculated by multiplying the intensity and degree of staining scores. The details of the solid tumour samples are listed in Supplementary Data 2. This study was approved by the Biomedical Research Ethics Review Committee of FJMU (FJMU200918), the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (MTCA, ECFAH of FMU [2015]084-2) and the Ethics Committee of Fujian Medical University Union Hospital (2017KY091). Due to the nature of the retrospective study, the need for informed consent was waived by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University and the Fujian Medical University Union Hospital. All ethical regulations relevant to human research participants were followed.
Western blotting (WB) and real-time quantitative PCR (RT‒qPCR)
WB was performed using the standard method, as reported previously59. An anti-GAPDH antibody was used as a loading control to normalize the levels of other proteins. All antibodies used are listed in Supplemental Table S1.
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into complementary DNA with a PrimeScript Reverse Transcriptase kit (Takara, Dalian, China). RT-qPCR was performed on a 7500 Real-Time PCR system with a SYBR Real-Time PCR kit (Takara). All primers used are listed in Supplemental Table S2. The relative gene expression level was calculated using the 2 − ΔΔCT method.
Construction of lentivirus containing the shTCP1 vector
Cells used for TCP1 knockdown were infected with lentiviruses expressing TCP1 shRNA or scrambled shRNA and selected with puromycin (2 μg/ml). The optimal sequences of TCP1 short hairpin RNA (shRNA) were as follows: TCP1-shRNA/F: 5’ CCGGGGTGTACAGGTGGTCATTATTCAAGAGATAATGACCACCTGTACACCTTTTTTG 3’; and TCP1-shRNA/R: 5’ AATTCAAAAAAGGTGTACAGGTGGTCATTATCTCTTGAATAATGACCACCTGTACACC 3’. A negative control sequence with no homology to the human genome was created as a scrambled sequence. The validated TCP1 shRNA and the scrambled control were subcloned and inserted into the pKLO.1 lentiviral vector, generated and transfected into HEK293T cells with helper plasmids (pVSVG, pMDL, and pREV) for 48–72 h. The supernatant containing the virus was collected and stored. Finally, freshly plated cells were infected with lentivirus, and the knockdown efficiency of the target genes was determined by WB or RT‒qPCR.
Cell proliferation assays
Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and cell colony formation assays. The MTT assay was performed using standard techniques, as previously reported60. For the cell colony formation assay, the number of colonies containing 50 or more cells was counted under a light microscope. The colony formation rate was calculated as the proportion of colonies/seeded cells.
Transwell assays
For the Transwell assays, 2 × 105 cells were suspended in 200 μl of serum-free medium and then seeded into the upper chamber without or with Matrigel for migration or invasion assay. The lower chamber was filled with 800 μl of the whole medium. Following incubation for 36 h, the cells that had migrated or invaded through the membrane were fixed and stained with 0.1% crystal violet and counted under a microscope.
Ubiquitination assay
Cells (transfected with scrambled shRNA and shTCP1) were transiently transfected with Flag-c-Myc and HA-ubiquitin using TurboFect transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions for 35 h. All cells were treated with MG132 for 1 h; subsequently, the cells were lysed with IP lysis buffer and boiled at 95 °C for 10 min. The supernatant was transferred to a new tube and incubated with a Flag-tagged antibody and protein A/G agarose to purify the transfected c-Myc protein. The precipitate was analysed by WB with anti-HA to detect the ubiquitinylated c-Myc protein.
Subcutaneous xenograft tumour model in nude mice
For xenograft experiments, the hepatocellular carcinoma and pancreatic ductal adenocarcinoma nude mouse models were constructed as previously described 61. Briefly, SMMC-7721, Huh7, or PANC-1 cells (2 × 106) stably expressing shTCP1 or scramble shRNA were subcutaneously injected into the bilateral upper flank regions of nude mice. Tumour growth was monitored every 3 days until the first mouse was sacrificed, and tumour volume was calculated with the following formula: volume = ½ × length × width2. When the tumour volume of the first mouse reached 1000 mm3, the mice were sacrificed, and the tumours were removed to calculate their size and weight. The process was approved by the Animal Ethics Committee of FJMU (IACUC-FJMU 2022-Y-0541) and in none of the experiments were the permitted tumour size limits exceeded.
In vivo metastatic assays in nude mice and bioluminescence imaging (BLI)
BxPC-3 and SMMC-7721 cells were transfected with lentivirus labelled with luciferase and shTCP1. BxPC-3 and SMMC-7721 cells stably expressing luciferase (2 × 106 cells) were suspended in 100 µl of PBS and injected into nude mice via the tail vein. Lung metastasis was monitored by BLI using an IVIS Spectrum In Vivo Imaging System (PerkinElmer, Santa Clara, CA, USA) starting 2 weeks after implantation. Fifteen minutes before BLI, the animals were injected intraperitoneally with d-luciferin (150 mg/kg). The experiments were performed every two weeks for 8 weeks.
Transgenic mice and primary hepatocarcinoma model
The mouse TCP1 gene is located on mouse chromosome 17, and the mouse ROSA26 gene is located on mouse chromosome 6. The “CAG-mouse TCP1 CDS-poly A” targeting vector was inserted into ROSA26 gene intron 1 to produce TCP1-KI mice. To engineer the targeting vector, homology arms were generated by PCR using a BAC clone from the C57BL/6 library as a template. Cas9 and gRNA were coinjected into fertilized eggs via a targeting vector. Cas9 specifically cuts the ROSA26 gene intron sequence under the guidance of gRNA, and then the “CAG-mouse TCP1 CDS-poly A” targeting vector is inserted into the ROSA26 gene intron by homologous recombination for KI mouse production. The pups were genotyped by PCR followed by sequencing analysis. Wild-type (WT) and TCP1-KI mice (C57BL/6J background) were purchased from Cyagen Biosciences Inc. (Suzhou, China). For the DEN-induced HCC model, 15-day-old male mice were intraperitoneally injected with DEN (dissolved in PBS, 25 mg/kg body weight). After 12 months, the mice were euthanized, and liver tissues were dissected and used for follow-up experiments. The number of tumour nodules, the ratio of liver weight, and the tumour volume were evaluated in a blinded manner between WT and TCP1-KI mice that were not randomly allocated. Then, HE, IHC, and WB assays were used to detect the related indexes using standard techniques.
Survival analysis
Survival analysis of tumour samples from the TCGA dataset based on the TCP1 expression level. The TCP1 expression level was merged with the clinical information to perform a Kaplan–Meier survival analysis using survival R package (version 0.4.9) and utilized survminer R package (version 3.7-0) to visualize the resulting survival curve.
Statistics and reproducibility
The findings were analysed using the statistical software SPSS for Windows, version 22.0, and the GraphPad Prism 5 software package. The Mann‒Whitney U test was used to test for differences in TCP1 expression between cancer tissues and their corresponding normal tissues. χ2 analysis or Fisher’s exact test was used to test for associations between TCP1 expression and clinicopathological parameters in patients with HCC or PDAC. Survival data were evaluated with the Kaplan–Meier method combined with the log-rank test. Pearson correlation analysis was applied to determine the relationship between TCP1 expression and tumour recurrence. Student’s t-test was used for the in vitro and in vivo experiments. One-way ANOVA was performed to compare the means of multiple groups. Statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significance. The data are shown as the means ± SD.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of the paper are present in the main text/or the supplementary information. The source data behind the graphs in the paper are available as Supplementary Data 1. The IHC score of clinical samples is available as Supplementary Data 2. All the public data used was downloaded from the TCGA dataset (https://portal.gdc.cancer.gov/) and gtex dataset (https://www.gtexportal.org/), and analysed using the R packages “survival” and “survminer”. The analysis process will be provided when available.
References
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Morimoto, R. I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 22, 1427–1438 (2008).
Yam, A. Y. et al. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 15, 1255–1262 (2008).
Marco, S., Carrascosa, J. L. & Valpuesta, J. M. Reversible interaction of beta-actin along the channel of the TCP-1 cytoplasmic chaperonin. Biophys. J. 67, 364–368 (1994).
Llorca, O. et al. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 402, 693–696 (1999).
Qian-Lin, Z. et al. Inhibition of cytosolic chaperonin CCTζ-1 expression depletes proliferation of colorectal carcinoma in vitro. J. Surg. Oncol. 102, 419–423 (2010).
Cui, X. et al. Overexpression of chaperonin containing TCP1, subunit 3 predicts poor prognosis in hepatocellular carcinoma. World J. Gastroenterol. 21, 8588–8604 (2015).
Huang, X. et al. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. Apmis 122, 1070–1079 (2014).
Guest, S. T. et al. Two members of the TRiC chaperonin complex, CCT2 and TCP1 are essential for survival of breast cancer cells and are linked to driving oncogenes. Exp. Cell Res. 332, 223–235 (2015).
Hallal, S. et al. Extracellular vesicles from neurosurgical aspirates identifies chaperonin containing TCP1 subunit 6 A as a potential glioblastoma biomarker with prognostic significance. Proteomics 19, e1800157 (2019).
Yang, J. et al. CCT α is a novel biomarker for diagnosis of laryngeal squamous cell cancer. Sci. Rep. 9, 11823 (2019).
Liu, Y. J., Chang, Y. J., Kuo, Y. T. & Liang, P. H. Targeting β-tubulin/CCT-β complex induces apoptosis and suppresses migration and invasion of highly metastatic lung adenocarcinoma. Carcinogenesis 41, 699–710 (2020).
Kasembeli, M. et al. Modulation of STAT3 folding and function by TRiC/CCT chaperonin. PLoS Biol. 12, e1001844 (2014).
Bassiouni, R. et al. Chaperonin containing TCP-1 protein level in breast cancer cells predicts therapeutic application of a cytotoxic peptide. Clin. Cancer Res. 22, 4366–4379 (2016).
Trinidad, A. G. et al. Interaction of p53 with the CCT complex promotes protein folding and wild-type p53 activity. Mol. Cell 50, 805–817 (2013).
Kim, A. R. & Choi, K. W. TRiC/CCT chaperonins are essential for organ growth by interacting with insulin/TOR signaling in Drosophila. Oncogene 38, 4739–4754 (2019).
Roh, S. H. et al. Contribution of the Type II Chaperonin, TRiC/CCT, to oncogenesis. Int. J. Mol. Sci. 16, 26706–26720 (2015).
Qian, E. N., Han, S. Y., Ding, S. Z. & Lv, X. Expression and diagnostic value of CCT3 and IQGAP3 in hepatocellular carcinoma. Cancer Cell Int. 16, 55 (2016).
Ying, Z. et al. CCT6A suppresses SMAD2 and promotes prometastatic TGF-β signaling. J. Clin. Investig. 127, 1725–1740 (2017).
Tang, N. et al. TCP1 regulates Wnt7b/β-catenin pathway through P53 to influence the proliferation and migration of hepatocellular carcinoma cells. Signal Transduct. Target Ther. 5, 169 (2020).
Zhang, S. et al. TCP1 expression alters the ferroptosis sensitivity of diffuse large B-cell lymphoma subtypes by stabilising ACSL4 and influences patient prognosis. Cell Death Dis. 15, 611 (2024).
Ray, D. et al. Inducing oncoprotein degradation to improve targeted cancer therapy. Neoplasia 17, 697–703 (2015).
Dang, C. V. MYC on the path to cancer. Cell 149, 22–35 (2012).
Gabay, M., Li, Y. & Felsher, D. W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 4, a014241 (2014).
Wirth, M., Mahboobi, S., Krämer, O. H. & Schneider, G. Concepts to target MYC in pancreatic cancer. Mol. Cancer Ther. 15, 1792–1798 (2016).
Wang, W. et al. SCP1 regulates c-Myc stability and functions through dephosphorylating c-Myc Ser62. Oncogene 35, 491–500 (2016).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Stokoe, D. et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 (1997).
Osaki, M., Oshimura, M. & Ito, H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 9, 667–676 (2004).
Cross, D. A. et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Sears, R. C. The life cycle of C-myc: from synthesis to degradation. Cell Cycle 3, 1133–1137 (2004).
Arnold, H. K. et al. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 28, 500–512 (2009).
Hydbring, P. et al. Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc. Natl Acad. Sci. USA 107, 58–63 (2010).
Liu, Y. J., Kumar, V., Lin, Y. F. & Liang, P. H. Disrupting CCT-β: β-tubulin selectively kills CCT-β overexpressed cancer cells through MAPKs activation. Cell Death Dis. 8, e3052 (2017).
Showalter, A. E. et al. Investigating chaperonin-containing TCP-1 subunit 2 as an essential component of the chaperonin complex for tumorigenesis. Sci. Rep. 10, 798 (2020).
Yao, L., Zou, X. & Liu, L. The TCP1 ring complex is associated with malignancy and poor prognosis in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 12, 3329–3343 (2019).
Gao, H. et al. Chaperonin containing TCP1 subunit 5 is a tumor associated antigen of non-small cell lung cancer. Oncotarget 8, 64170–64179 (2017).
Sternlicht, H. et al. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc. Natl Acad. Sci. USA 90, 9422–9426 (1993).
Vallin, J. & Grantham, J. The role of the molecular chaperone CCT in protein folding and mediation of cytoskeleton-associated processes: implications for cancer cell biology. Cell Stress Chaperones 24, 17–27 (2019).
Feldman, D. E., Thulasiraman, V., Ferreyra, R. G. & Frydman, J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol. Cell 4, 1051–1061 (1999).
Weng, H., Feng, X., Lan, Y. & Zheng, Z. TCP1 regulates PI3K/AKT/mTOR signaling pathway to promote proliferation of ovarian cancer cells. J. Ovarian Res. 14, 82 (2021).
Chen, X. et al. TCP1 increases drug resistance in acute myeloid leukemia by suppressing autophagy via activating AKT/mTOR signaling. Cell Death Dis. 12, 1058 (2021).
Sherr, C. J. Cancer cell cycles. Science 274, 1672–1677 (1996).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Gurel, B. et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol. 21, 1156–1167 (2008).
Williams, K. et al. Unopposed c-MYC expression in benign prostatic epithelium causes a cancer phenotype. Prostate 63, 369–384 (2005).
Wonsey, D. R., Zeller, K. I. & Dang, C. V. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc. Natl Acad. Sci. USA 99, 6649–6654 (2002).
Koh, C. M. et al. MYC and prostate cancer. Genes Cancer 1, 617–628 (2010).
Miller, D. M. et al. c-Myc and cancer metabolism. Clin. Cancer Res. 18, 5546–5553 (2012).
Wang, Z. et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell 33, 706–720.e9 (2018).
Sviripa, V. M. et al. Halogenated diarylacetylenes repress c-myc expression in cancer cells. Bioorg. Med. Chem. Lett. 24, 3638–3640 (2014).
Akinyeke, T. et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis 34, 2823–2832 (2013).
Ramsay, G., Evan, G. I. & Bishop, J. M. The protein encoded by the human proto-oncogene c-myc. Proc. Natl Acad. Sci. USA 81, 7742–7746 (1984).
Tsai, W. B. et al. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 72, 2622–2633 (2012).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 101, 9085–9090 (2004).
Bowles, D. W. & Jimeno, A. New phosphatidylinositol 3-kinase inhibitors for cancer. Expert Opin. Investig. Drugs 20, 507–518 (2011).
Pal, S. K., Reckamp, K., Yu, H. & Figlin, R. A. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin. Investig. Drugs 19, 1355–1366 (2010).
Chin, L., Andersen, J. N. & Futreal, P. A. Cancer genomics: from discovery science to personalized medicine. Nat. Med. 17, 297–303 (2011).
Lian, X. et al. Pin1 inhibition exerts potent activity against acute myeloid leukemia through blocking multiple cancer-driving pathways. J. Hematol. Oncol. 11, 73 (2018).
Yan, X. et al. MicroRNA-140-5p inhibits hepatocellular carcinoma by directly targeting the unique isomerase Pin1 to block multiple cancer-driving pathways. Sci. Rep. 7, 45915 (2017).
Chen, L. et al. Targeting PIN1 exerts potent antitumor activity in pancreatic ductal carcinoma via inhibiting tumor metastasis. Cancer Sci. 110, 2442–2455 (2019).
Acknowledgements
We are grateful to Professor Kun Ping Lu and Tae Ho Lee for reading the manuscript and making valuable suggestions. We also thank Zi Yin, Donglan Yu, Ruijuan Yang, Xiao Xu, Zhiwei Tu, Zhentao Zhang, Lu Ye, Junfeng Kang and Zhuo Tao Qiu for providing help with the experiments. The work was supported by the National Natural Science Foundation of China (81171972) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (2017Y9054). Additionally, we thank the participating patients for providing the pathological samples.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.Z.C., H.K.L., Y.W.; Cell experiments: H.K.L., L.Y.C., Y.Y.J., X.C.C., N.J.M., J.C. and Y.P.L.; Animal experiments: Y.W., X.C.C., F.Y., X.Y.L., S.M.X., H.X.B.; Acquisition of data: Y.H.Y., W.X.X., X.H.L.; Analysis and interpretation of data: L.Y.C., Y.Y.J., X.X.W.; Investigation: all authors; Supervision: L.Y.C., Y.Y.J., X.X.W.; Writing-original draft: H.K.L., L.Y.C., Y.Y.J. and Y.W.C.; Writing—review & editing: H.K.L., Y.W.C. and Y.Z.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Hetong Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Johannes Stortz.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, H., Chen, L., Chen, Y. et al. TCP1 promotes the progression of malignant tumours by stabilizing c-Myc through the AKT/GSK-3β and ERK signalling pathways. Commun Biol 8, 563 (2025). https://doi.org/10.1038/s42003-025-07867-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07867-6