Abstract
Norepinephrine (NE) is mainly released by sympathetic nerve terminals to act on organs and tissues. After corneal epithelial debridement, we found that the sympathetic nerve fibers penetrated the limbus and regenerated toward the cornea within 24 h post-wounding. Topical NE application recapitulated the characteristics of delayed corneal epithelial wound healing and nerve regeneration in healthy mice, accompanied by the partial depletion of multiple neurotrophins, such as nerve growth factor and glial cell-derived nerve growth factor. Moreover, the diabetes mellitus (DM) mice exhibited corneal sensory nerve dysfunction and increased plasma and corneal NE contents, which were rescued by 6-hydroxydopamine (6-OHDA) and bretylium. In the cell culture model, the conditioned medium of NE-treated corneal epithelial cells inhibited trigeminal ganglion (TG) neurite outgrowth, which was reversed by the β2 adrenergic receptor (ADRB2) antagonist, but not by the β1 adrenergic receptor (ADRB1) antagonist. Topical application of the ADRB2 antagonist recovered the expression of corneal neurotrophins, and promoted corneal epithelial and nerve regeneration in DM mice. Taken together, the NE-ADRB2 axis regulates corneal neurotrophin expression and nerve regeneration in mice. Topical application of the ADRB2 antagonist may represent a promising therapeutic strategy for diabetic corneal sensory nerve dysfunction.
Similar content being viewed by others
Introduction
The sympathetic nervous system (SNS) regulates various physiological processes infight-or-flight mode to maintain bodily functions and homeostasis1. The cornea is the most densely innervated tissue, in which the sensory nerves are predominantly derived from trigeminal ganglion (TG), the sympathetic nerves are derived from the superior cervical ganglion and the parasympathetic nerves are derived from the ciliary ganglion2. Upon perceiving threats to homeostasis, the SNS mainly releases norepinephrine (NE) from sympathetic nerve terminals to act on organs and tissues in response to stressors. The endogenous ligands of catecholamine are widely expressed widespread in a multitude of cell types and tissues, which include α adrenoreceptor subtypes (α1A, α1B, α1D, α2A, α2B, and α2C) and β adrenoreceptor subtypes (β1, β2, and β3)3. In the cornea, β2 adrenergic receptor (ADRB2) is dominant among the β adrenoreceptor subtypes4. The activation of SNS induces a local inflammatory reaction by stimulating CD64+CCR2+ macrophages and inhibits corneal wound healing by activating the ADRB2 of the corneal epithelial cells4. Conversely, the parasympathetic nervous system exerts the opposite effects compared with the SNS on corneal epithelial wound healing. The activation of parasympathetic nerves inhibits local inflammation by stimulating CD64+CCR2- macrophages and promotes corneal wound healing through the α-7 nicotinic acetylcholine receptor (α7nAChR) of the corneal epithelial cells4. In the clinic, both type 1 and type 2 diabetic patients also exhibit changes in the sympathetic and parasympathetic nervous system5,6,7,8, named as diabetic autonomic neuropathy (DAN)9. The DAN was reported to be involved in multiple diabetic complications, such as the gastrointestinal, genitourinary, cardiovascular, sudomotor and neuroendocrine systems10.
The corneal nerves maintain corneal homeostasis and regenerative capacity by secreting various neurotransmitters and neuropeptides11,12,13,14,15,16,17. The sensory nerves secrete substance P (SP)11,12 and calcitonin gene-related peptide (CGRP)16, and parasympathetic nerves secrete vasoactive intestinal polypeptide (VIP)14. Corneal epithelium also expresses multiple neurotrophic factors, such as nerve growth factor (NGF), glial cell–derived nerve growth factor (GDNF), mesencephalic astrocyte-derived neurotrophic factor (MANF), and ciliary neurotrophic factor (CNTF) and contributes to maintenance of corneal nerve fibers18,19,20,21,22. In diabetes mellitus (DM), the patients develop diabetic peripheral neuropathy23,24,25,26, including diabetic keratopathy in the cornea, which exhibits reduced corneal nerve density and sensitivity, as well as delayed wound healing17,27,28. However, the pathogenic mechanism of reduced neurotrophins in diabetic cornea remains undefined. Our previous studies confirmed that the expression of neurotrophins in diabetic corneas was decreased, such as NGF, GDNF, and CNTF. Accordingly, exogenous supplementation of these neurotrophins could promote corneal nerve regeneration and sensation recovery in DM mice20,22. Although the activation of SNS and the reduction of neurotrophins in diabetic corneas have been well studied29, the relationship between the sympathetic neurotransmitter NE and corneal neurotrophin secretion remains unknown.
To address this issue, we focused on the regulation and mechanism of NE on corneal sensory nerve regeneration in healthy and diabetic mice. Through both an in vivo animal model and an in vitro cell culture model, we demonstrated that elevated NE impaired corneal nerve regeneration via the ADRB2-mediated neurotrophin depletion. Moreover, we confirmed that the plasma and corneal NE contents were elevated significantly in DM mice compared to healthy mice after corneal injury. Accordingly, sympathetic neurotoxin 6-hydroxydopamine (6-OHDA) and ADRB2 antagonist ICI 118, 551 recovered the expression levels of corneal neurotrophins and promoted corneal nerve regeneration, sensation recovery and wound healing in DM mice.
Results
NE modulates corneal epithelium wound healing and nerve regeneration
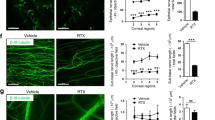
To visualize the sympathetic innervations in mouse cornea, corneal nerve fibers and sympathetic nerve fibers were labeled with α-βIII tubulin and α-tyrosine hydroxylase (TH) antibodies, respectively. Our results demonstrated that, the corneal sympathetic nerve fibers in C57BL/6 J mice are confined to the limbal region. However, these fibers penetrated the limbus and regenerated toward the central cornea within 24 h post-wounding (Fig. 1A). This suggested that the sympathetic nerve fibers, which primarily secreted NE, might also play a role in corneal wound healing. To investigate the direct effect of NE on mouse corneal wound healing, we applied 10 mM NE topically four times daily immediately after epithelial debridement in C57BL/6 J mice. As shown in Fig. 1B, the NE-treated mice displayed a decreased rate of epithelial regeneration at 24 h compared to the control mice receiving vehicle treatment (Fig. 1B, C). Furthermore, corneal nerve density and sensitivity remained significantly reduced in the NE-treated mice (Fig. 1D–F).
A The corneal nerve fibers were labeled with α-βIII tubulin antibody and sympathetic nerve fibers were stained by α-TH antibody in C57BL/6 J mice before and after corneal epithelial debridement for 24 h. B, C C57BL/6 J mice were performed with corneal epithelial debridement and topically applied with the eye drop containing 10 mM NE. Mouse corneal epithelial defects were monitored with fluorescein staining and calculated with Image J software (n = 5 mice per group). D, E Mouse corneal nerves were stained by βIII-tubulin antibody and the subbasal nerve fibers were counted with Image J software at 48 h post wounding (n = 4 corneas per group). F Corneal sensations were measured with a Cochet-Bonnet esthesiometer, conducted two days post wounding. Data are representative of the mean ± SD. **p < 0.01, ***p < 0.001.
NE regulates the neurotrophin expression of corneal epithelial cells
Our previous study revealed that the corneal epithelium expressed neurotrophins NGF and GDNF, which promoted corneal nerve regeneration in both the healthy and the DM mice. Moreover, NGF and GDNF expression was significantly attenuated in the regenerating corneal epithelium of DM mice when compared with healthy mice20. Combined with the correlation of NE-treated and DM mice in delayed corneal wound healing, we hypothesized that the elevated NE might be involved in the regulation of neurotrophins expression of the corneal epithelium.
To demonstrate the direct regulation of NE on the expression of neurotrophins derived from the corneal epithelium, we treated the cultured human corneal epithelial cells (HCECs) with different concentrations of NE. Similar to the in vivo results, NE treatment reduced the NGF mRNA transcriptions of HCECs in a dose-dependent manner (Fig. 2A). More interestingly, the expressions of other neurotrophins, such as GDNF, CNTF, neurotrophic factor-3 (NTF-3), neurotrophic factor-4/5 (NTF-4/5), and Slit2 were attenuated, while the repulsive axon guidance factor Sema3a was increased with the 10 μM NE treatment (Fig. 2B). The decreased NGF and GDNF expression in NE-treated HCECs was confirmed by qPCR, ELISA and immunofluorescence staining (Fig. 2C–F). These results indicated the effect of NE on the neurotrophins expression of corneal epithelial cells in vitro.
A The NGF mRNA expression of HCECs with different NE concentration treatments. B The expression of various neurotrophins in HCECs with 10 μM NE treatment was detected with qPCR. The NGF and GDNF expression of HCECs treated with 10 μM NE were detected with qPCR (C), ELISA (D) and immunofluorescence staining (E). F The fluorescence intensity of NGF and GDNF in HCECs treated with 10 μM NE was quantified by Image J software. Corneal NGF and GDNF expressions were measured with qPCR (G) and ELISA (H). I Immunofluorescence staining of NGF and GDNF in the cornea. J The fluorescence intensity of NGF and GDNF was quantified by Image J software. Data are representative of the mean ± SD. **p < 0.01, ***p < 0.001.
Furthermore, the NGF and GDNF expression of NE-treated mice was detected by qPCR, ELISA and immunofluorescence staining. The qPCR results showed that the mRNA transcripts of NGF and GDNF were significantly decreased after NE treatment (Fig. 2G). ELISA results also indicated that the protein concentration of NGF and GDNF was significantly downregulated in NE-treated mice, which decreased from 427.99 ± 24.02 pg/mg and 361.01 ± 47.20 pg/mg to 307.30 ± 20.51 pg/mg and 198.31 ± 5.61 pg/mg, respectively (Fig. 2H). The immunofluorescence staining further confirmed that the NE-treated mice exhibited weaker staining of NGF and GDNF in the corneal epithelium compared to the control mice (Fig. 2I, J). Collectively, the in vitro models confirmed that NE directly regulates neurotrophin expression.
Sympathetic intervention recovered NGF and GDNF expression in diabetic corneas
To evaluate the activation changes of SNS, we adapted type 1 DM mice induced by intraperitoneal streptozotocin (STZ) injection according to our previous description30, with age-matched healthy mice as controls. After 16 weeks of final injections, the blood glucose of DM mice was 30 mmol/L compared with 7 mmol/L of age-matched healthy mice (Fig. 3A). The central corneal epithelium of the control and DM mice was then debrided as a corneal wound healing model. As expected, DM mice showed a delayed epithelial regeneration rate with larger defects than healthy mice (Fig. 3B, C). Whole-mounted corneal staining exhibited a sparse subbasal nerve plexus and reduced nerve density in DM mice compared to healthy mice (Fig. 3D, E). In line with delayed nerve regeneration, corneal sensitivity was similarly attenuated in DM mice from 3 to 10 days post-wounding (Fig. 3F). Moreover, we collected mouse blood and corneal samples, and compared plasma and corneal NE contents between DM and healthy mice. ELISA measurement revealed that both plasma and corneal NE concentrations were significantly elevated in DM mice at 12 h and 24 h post-wounding (Fig. 3G, H). Collectively, these results suggest that the elevated plasma and corneal NE contents are accompanied by delayed corneal wound healing in DM mice.
The corneal epithelium was debrided in DM mice after 16 weeks of STZ injection, with the age-matched mice as controls. A After 16 weeks of final STZ injection, the blood glucose was measured and compared with age-matched healthy mice (n = 16 mice per group). B Mouse corneal epithelial wound healing was monitored with fluorescein staining. C Corneal epithelial defects were calculated with Image J software (n = 5 mice per group). D Mouse corneal nerves were stained with Alexa Fluor® 488-labeled α-βIII-tubulin antibody. E The subbasal nerve fibers were counted with Image J software (n = 4 corneas per group). F Corneal sensations were measured with a Cochet-Bonnet esthesiometer and compared between DM and healthy mice. G, H Mouse plasma and corneal NE levels were measured with ELISA kits at 12 h and 24 h after corneal wounding (n = 4 mice per group for plasma, n = 4 corneas per group for cornea). Data are representative of the mean ± SD. **p < 0.01, ***p < 0.001, ****P < 0.0001.
To demonstrate the effects of elevated NE contents on delayed diabetic corneal wound healing, we ablated the sympathetic nervous system of DM mice with intraperitoneal injections of 6-OHDA, according to previous descriptions4. The healthy, DM, and 6-OHDA-treated DM mice were further evaluated with a corneal epithelial debridement model. As expected, the elevation of plasma and corneal NE was inhibited after 6-OHDA treatment in the DM mice (Fig. 4A, B). According to fluorescein staining, 6-OHDA treatment caused rapid epithelial wound healing in DM mice (Fig. 4C), as confirmed by the quantification of the corneal epithelial defect area (Fig. 4D). Then, we harvested the regenerating cornea and evaluated NGF and GDNF expressions in healthy, DM and 6-OHDA-treated DM mice at 48 h after epithelial debridement. The qPCR and ELISA results showed that the mRNA transcripts and protein levels of corneal NGF and GDNF were reduced in DM mice, but significantly recovered in 6-OHDA-treated DM mice (Fig. 5A, B). Immunofluorescence staining further confirmed that the 6-OHDA-treated DM mice exhibited apparently stronger staining of NGF and GDNF in the corneal epithelium compared to the DM mice (Fig. 5C, D). Moreover, corneal nerve regeneration was also accelerated in the 6-OHDA-treated DM mice when compared with the DM mice 48 h post-wounding (Fig. 5E, F), accompanied by improved recovery of corneal sensitivity (Fig. 5G).
DM mice were intraperitoneally injected with 6-OHDA and subsequently debrided the central corneal epithelium. A, B Mouse plasma and corneal NE concentrations were measured with ELISA kits at 12 h and 24 h post wounding (n = 3 mice per group). C Mouse corneal epithelial wound healing was monitored with fluorescein staining. D Corneal epithelial defects were calculated with Image J software (n = 5 mice per group). Data are representative of the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
DM mice were intraperitoneally injected with 6-OHDA and subsequently debrided the central corneal epithelium. The corneas of healthy, DM, and 6-OHDA-treated DM mice were collected at 48 h after epithelial debridement. A NGF and GDNF mRNA transcripts were monitored with qPCR (n = 3 mice per group). B NGF and GDNF protein levels were measured with an ELISA (8 corneas pooled per group). C Immunofluorescence staining of NGF and GDNF in the cornea. D The fluorescence intensity of NGF and GDNF in the corneal epithelium was quantified by Image J software. E Mouse corneal nerves were stained with the Alexa Fluor® 488-labeled α-βIII-tubulin antibody at 48 h post wounding. F The subbasal nerve fibers were counted with Image J software (n = 4 corneas per group). G Corneal sensations were measured with a Cochet-Bonnet esthesiometer (n = 4 mice per group). Data are representative of the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To reduce the over-release of NE in DM mice, bretylium was topically applied. The results demonstrated that bretylium not only accelerated corneal wound healing (Fig. S1A, B), bult also promoted the recovery of corneal nerve density and sensitivity in DM mice (Fig. S1C–E). Therefore, these findings suggest that sympathetic intervention promoted the regeneration of the corneal epithelium and nerves in DM mice.
ADRB2 mediates the regulation of NE on corneal neurotrophin expression
To explore the major receptors of NE and the regulation of neurotrophin expression in the corneal epithelium, we detected the expression levels of various adrenergic receptor types in mouse corneal epithelium. The results confirmed that ADRB2 was the dominant receptor in the corneal epithelium, surpassing ADRB1 and other receptors (Fig. 6A). Therefore, we added the ADRB2 antagonist ICI 118, 551 (ICI) or the ADRB1 antagonist atenolol (ATE) to the NE-treated HCECs or murine corneal epithelial stem/progenitor cell line (TKE2) cells. The results showed that the mRNA transcripts and protein levels of NGF and GDNF were significantly recovered with the addition of ICI 118, 551, but not atenolol (Fig. 6B–E). Moreover, the conditioned media of the TKE2 cells were collected and incubated with freshly-isolated mouse TG neurons (Fig. 6F). As shown in Fig. 6G, H, neurite outgrowth was attenuated when incubated with the conditioned medium of NE-treated cells, which was reversed by the conditioned medium of NE-treated cells in the presence of ICI 118, 551, but not atenolol. Overall, these results indicate that ADRB2 mediates the regulation of NE on NGF and GDNF expression in the corneal epithelium.
A Relative expression of ADRs in the mouse cornea. B–E NGF and GDNF mRNA transcripts and protein levels were detected with qPCR and ELISA in HCECs treated with PBS, NE, NE and atenolol (ATE), and NE and ICI 118, 551 (ICI). F Scheme of indirect cell culture of TKE2 cells and TG neurons. G Representative TG neurite outgrowth incubated with the various conditioned media of TKE2 cells treated with PBS, NE, NE and ATE, and NE and ICI. The cultured TG neurons were stained with Alexa Fluor® 488-labeled βIII-tubulin antibody. H The TG neurite outgrowth was analyzed with Neuro J software. Data are representative of the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Topical application of ICI 118, 551 promotes diabetic corneal wound healing
Based on the regulation of the ADRB2 antagonist ICI 118, 551 on neurotrophins expression, we adopted the topical application of 10 μM ICI 118, 551 four times per day immediately after epithelial debridement in DM mice. The results showed that topical ICI 118, 551 applications significantly promoted diabetic corneal epithelial healing (Fig. 7A, B) and subbasal nerve regeneration (Fig. 7C, D), as well as rapid recovery of corneal sensitivity (Fig. 7E), when compared with vehicle controls. Moreover, the expression of NGF and GDNF was also elevated compared to those without ICI 118, 551 treatments, as confirmed by qPCR and immunofluorescence staining (Fig. 7F-H). The results suggest that the ADRB2 antagonist promotes corneal wound healing and increases neurotrophin levels in DM mice.
DM mice were performed with corneal epithelial debridement and topically applied with the eye drop containing 10 μM ICI 118, 551 (ICI). A, B Mouse corneal epithelial defects were monitored with fluorescein staining and calculated with Image J software (n = 5 mice per group). C, D Mouse corneal nerves were stained and the subbasal nerve fibers were counted with Image J software at 48 h post wounding (n = 4 corneas per group). E Corneal sensations were measured with a Cochet-Bonnet esthesiometer at 48 h post wounding (n = 5 mice per group). Corneal NGF and GDNF expressions were detected with qPCR (n = 3 mice per group) at 48 h post wounding (F) and immunofluorescence staining (G). H The fluorescence intensity of NGF and GDNF in the corneal epithelium was quantified by Image J software. Data are representative of the mean ± SD. *p < 0.05, **p < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In this study, we demonstrated the role and mechanism of the sympathetic neurotransmitter NE in the pathogenesis of corneal neurotrophin expression and nerve regeneration in an in vivo animal model and an in vitro cell culture model. The DM mice exhibited increased plasma and corneal NE contents, which reduced corneal epithelial and nerve regeneration by depletion of multiple neurotrophins, such as NGF and GDNF. Moreover, 6-OHDA and the ADRB2 antagonist ICI 118, 551 reversed delayed corneal wound healing and nerve regeneration in DM mice by increasing neurotrophins expression. Taken together, the NE-ADRB2 axis regulates corneal neurotrophin expression and nerve regeneration in mice. Inhibition of the NE-ADRB2 axis may provide a potential therapeutic strategy for the treatment of diabetic corneal sensory nerve dysfunction.
According to the recent study, the cornea is also under dual control of the sympathetic and parasympathetic nervous systems4,5,6,7,8. The sympathetically released NE is involved in the wound repair process through different kinds of immune cell populations. From the perspective of inflammation with corneal epithelial regeneration, Xue et al. found that sympathetic nerve activation impairs corneal wound healing by regulating different types of macrophages, while parasympathetic nerves exert opposite effects4. Moreover, the degranulation of mast cells is crucial for controlling inflammation and promoting corneal wound healing. In a study by Li et al., sympathetic nerve activation inhibits mast cell degranulation, whereas parasympathetic nerve activation enhances the degranulation31. Basic and clinical studies have shown abnormal SNS activation in diabetic models and patients. In the bladder base of STZ-induced type 1 DM rats for 4–8 weeks, the NE concentration was significantly higher than in the control rats, which might be required to adapt to the increased urine volume32. In the cardiac tissue and plasma of STZ rat models fed a high-fat diet, the NE levels were found to be 1.2-fold and 2.5-fold elevated, respectively, and the NE reuptake transporter was found to have a 17% reduction. However, there was no change in sympathetic nerve density, which indicated that sustained hyperglycemia resulted in dysregulation of SNS signaling33. In the clinic, the plasma concentrations of NE were higher in diabetic patients compared with non-diabetic patients, which is correlated with SNS activation5. Moreover, SNS activation or NE administration in diabetic Apoe–/– mice promoted the proliferation of granulocyte macrophage progenitors and myeloid cell development through ADRB2, which accelerated atherosclerosis in diabetes5. Furthermore, 6-OHDA treatment, which blocks the SNS, could diminish inflammation and improve the features of atherosclerotic lesions in DM mice by reducing splenic myeloid cells and monocyte numbers5. Our results demonstrated that blockades of SNS or ADRB2 by 6-OHDA or ICI 118, 551 reversed corneal epithelial and nerve regeneration.
The clinical manifestations of diabetic sensory nerve dysfunction include decreased sensory nerve density and corneal sensitivity, delayed epithelial regeneration, superficial punctate keratopathy and even corneal erosion34. Accumulating evidence has revealed the pathogenesis. Hyperglycemia impairs the corneal nerves’ secretion of neuropeptides, such as NGF and GDNF20, and promotes the generation of reactive oxygen species which causes mitochondrial damage35. Hyperglycemia also induces the accumulation of advanced glycation end products, which further leads to the generation of oxygen radicals and the release of pro-inflammatory cytokines36,37. Moreover, abnormality was observed in both corneal nerves and corneal epithelial cells including basal cells and limbal stem cells in DM38. Corneal wound healing requires precise regulation of corneal nerves and epithelial cells. The corneal nerves exert important neurotransmitters on the corneal epithelium, while corneal epithelial cells secrete neurotrophins to maintain the survival and extension of the corneal nerves39,40. Notably, corneal nerve regeneration is also positively influenced by the inflammatory process after corneal injury. Neurotrophins promote corneal nerve growth via CCR6+ IL-17+ γδ T cell-dependent release of VEGF-A, which is a trophic factor for corneal nerves41,42. Localized macrophages release nerve regrowth-promoting growth factors and immunosuppressive cytokines, such as TAFA4 and IL-104,43, following tissue injury. In addition, mast cells located in the vascular network around the corneal rim have great potential to produce NGF and other neurotrophic factors44,45, which indicates that mast cells are intimately involved in corneal nerve regeneration. In the current study, we found that the sympathetic neurotransmitter NE directly inhibited the expression of neurotrophins through ADRB2 of the corneal epithelial cells in DM mice, which is similar to severe heart failure in that a high plasma NE concentration causes the reduction of myocardial NGF46.
As it is the most abundant sensory nerve tissue in the body, the traditional approach to corneal keratopathy treatment is to target the sensory nerve. Topical application of NGF represents a promising strategy in nerve regeneration47, which has been clearly demonstrated in neurotrophic keratitis, diabetic neuropathy, and toxic neuropathy18,48,49. Currently, open-label studies that evaluated the efficacy of NGF in neurotrophic keratopathy patients at stages 2 and 3, show improvement in corneal healing, sensitivity, tear function, and visual acuity in most patients without the development of circulating antibodies to NGF18,47,49. NGF has received marketing authorization in Europe and the USA for treating neurotrophic keratopathy50. According to the current research, SNS activation along with reduced levels of NGF in the cornea have found in DM20,51. However, the understanding of DAN in corneal keratopathy remains unknown. Our study demonstrates, for the first time, the role and mechanism of sympathetic nerves in the neurotrophin secretion of the corneal epithelium in DM. We confirmed that the activation of sympathetic nerves impaired NGF and GDNF expression in the corneal epithelial cells of DM mice. Moreover, the inhibitory effect of NE on corneal epithelial cells could be reversed by 6-OHDA or ICI 118, 551 through inactivating the SNS or blocking the ADRB2 of corneal epithelial cells respectively, which promoted the regeneration of corneal nerves. These results suggest a novel approach for NGF and GDNF therapy in diabetic corneal sensory dysfunction.
β-blockers play a crucial role in the treatment of open-angle glaucoma or ocular hypertension, effectively reducing intraocular pressure by decreasing aqueous humor production and modulating its outflow52,53. However, topical application of β-blockers may negatively impact goblet cell density, corneal tear film, and the corneal epithelial barrier, heightening the risk of patients developing superficial punctate keratitis, dry eye syndrome, and conjunctivitis. Moreover, β-blockers may also cause systemic side effects, such as bradycardia, hypotension, and respiratory dysfunction54,55,56,57,58. Given these potential side effects, dosage becomes particularly critical when using ADRB2 antagonists. Our study has shown that a low dose of ICI 118,551 notably promotes corneal epithelial wound healing and nerve regeneration. However, a higher dose of ICI 118,551 was observed to impair corneal wound healing59. Consequently, it is imperative to weigh the potential side effects and risks carefully when using ADRB2 antagonists for corneal nerve regeneration.
In conclusion, our study demonstrated that the NE-ADRB2 axis regulates corneal neurotrophin expression and nerve regeneration. Topical application of the ADRB2 antagonist may represent a promising therapeutic strategy for the treatment of diabetic corneal sensory nerve dysfunction.
Methods
Animal models
Adult male C57BL/6 J mice (8-week-old) were purchased from the Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). We have complied with all relevant ethical regulations for animal use. All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the ethics committee of the Eye Institute of Shandong First Medical University. The animals were housed in a standard 12-h light/12-h dark cycle in the animal center. Type 1 DM mice were induced by intraperitoneal injection of 50 mg/kg STZ (Sigma Aldrich, St. Louis, MO) for five consecutive days according to our previous descriptions12. Blood glucose levels higher than 16.7 mmol/L were considered as DM and these mice were used after 16 weeks of finial STZ injection.
Corneal epithelial wound healing
The C57BL/6 J mice were anesthetized by an intraperitoneal injection of pentobarbital sodium (70 mg/kg) followed by topically anesthetized with 2% procaine hydrochloride, and all mice survived. The central corneal epithelium of right eye was marked with 2 mm trephine and debrided with an Alger brush II rust ring remover (Alger Co, Lago Vista, TX). Then, levofloxacin eye ointment was subsequently used to avoid infection. The defects of corneal epithelium were monitored under a BQ900 slit lamp (Haag-Streit, Bern, Switzerland) at 0, 24, 36, and 48 h after instilling 0.25% sodium fluorescein.
Corneal sensitivity
Corneal sensitivity was measured using a Cochet-Bonnet esthesiometer (Luneau Ophtalmologie, Chartres Cedex, France) in unanesthetized control, DM, NE-treated mice, 6-OHDA (Sigma, St. Louis, MO)-treated and ICI 118, 551 (Med Chem Express, Princeton, NJ)-treated DM mice at the indicated time periods. The corneas were measured with the beginning of the full length (6 cm) of a nylon filament and shortened by 5 mm until a blink response was found. The corneal sensitivity threshold was calculated as the mean of three determinations.
Pharmacological autonomic agonism and antagonism
To observe the effects of NE on corneal nerve regeneration, the mice received a 5 μL eye drop containing a 10 mM DL-NE hydrochloride (Aladdin, Shanghai, China) dissolved in 0.9% saline four times per day for continuous two days after epithelial wounding, while the mice received 0.9% saline as vehicle control. For chemical sympathectomy, the DM mice were intraperitoneally injected with 6-OHDA (100 mg/kg) for four continuous days. Control mice received the same volume of saline containing 0.02% ascorbic acid. For two days after the final injection, the mice were used for corneal epithelial wounding. To inhibit the release of NE from adrenergic nerve endings, the DM mice were treated topically with a 5 μL eye drop containing 50 μM bretylium tosylate (six times a day, Med Chem Express, Princeton, NJ). To observe the effect of inhibiting ADRB2 on corneal nerve regeneration, the DM mice were topically treated with 5 μL eye drop containing 10 μM ADRB2 antagonist ICI 118, 551 four times a day for continuous two days after corneal epithelial wounding.
Cell culture and treatment
HCECs and TKE2 cells were kindly provided by Choun-Ki Joo (The Catholic University of Korea, Seoul, Korea) and Dr. Tetsuya Kawakita (Keio University, Tokyo, Japan). The cells were cultured according to previous studies60,61. After starvation for 12 h, the HCECs cells were treated with 0.1–500 μM NE, 10 μM NE and 50 μM Atenolol (Med Chem Express), or 10 μM NE and 50 μM ICI 118, 551 for 24 h and TKE2 cells were incubated with 10 μM NE, 10 μM NE and 20 μM Atenolol, 10 μM NE and 20 μM ICI 118, 551 for 24 h.
TG neuron culture and treatment
The TG neurons were harvested and digested with papain and collagenase/dispase II, followed by percoll gradients separation as described previously20. Then, the neurons were cultured in neurobasal-A medium (Life Technologies, Grand Island, NY, USA) with B27 supplement (Life Technologies) on laminin-coated slides (Thermo Scientific, Waltham, MA, USA) overnight. After attachment, the neurons were incubated with medium containing 50% neurobasal-A medium and 50% conditioned medium of TKE2 cells for 24 h. Finally, the neuron outgrowth was analyzed and calculated with the quantitative nerve tracing software Neuro J (A plug-in for Image J, National Institutes of Health, Bethesda, MA).
Immunofluorescence staining
For corneal whole-mount staining, the corneas of mouse eyeballs were dissected and fixed in Zamboni’s fixative for 4 h at 4 °C. Then, the corneas were permeabilized and blocked by PBS with 0.1% TritonX-100 and 5% bovine serum albumin for 2 h, and subsequently incubated with Alexa Fluor® 488 conjugated βIII-tubulin mouse monoclonal antibody (Millipore, Darmstadt, Germany) or α-TH antibody (Sigma Aldrich) overnight at 4 °C and the flat mounts were examined with laser scanning confocal microscopy (Carl Zeiss, Germany). The neurite outgrowth of cultured TG neurons was fixed by 4% paraformaldehyde for 15 min and permeabilized with 0.1% TritonX-100 in PBS for 15 min, followed by 5% bovine serum albumin blocked for 1 h. Then, the neurons were incubated with Alexa Fluor® 488 conjugated βIII-tubulin rabbit polyclonal antibody (Merck) overnight at 4 °C. The staining was examined using an Eclipse TE2000-U microscope (Nikon, Tokyo, Japan). Corneal nerve fiber density was determined through the analysis of images obtained from whole-mount corneas. The images were initially converted to 8-bit gray scale. Then, the areas occupied by nerve terminals were measured with the ‘analyze particles’ tool of Image J software62. The sub-basal nerve plexuses within the observed region were semiautomatically traced, and the total area was subsequently calculated using the Image J software.
For immunofluorescence staining, the frozen sections (7 μm) or HCECs were fixed, permeabilized and blocked with the same method as cultured TG. Then, the sections and cells were incubated with α-NGF (Abcam, Cambridge, MA) and α-GDNF (Abcam) antibodies followed by fluorescein-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. The staining was observed and captured with Echo Revolve microscope (Echo Laboratories, San Diego, California) after staining with DAPI (Solarbio, Beijing, China).
ELISA analysis
To determine the NE level of control, DM, and 6-OHDA-treated DM mice, the mouse blood and corneas were collected at 12 h and 24 h after corneal epithelium scraped and the NE concentration was measured by the epinephrine/norepinephrine ELISA kit (Abnova, Taipei, China) according to the manufacturer’s protocol. For the evaluation of NGF and GDNF levels, the medium of HCECs was collected and measured using human NGF (Proteintech, Chicago, IL), and human GDNF (Abcam) ELISA kits according to the manufacturer’s guidance. Eight corneas of NE-treated, DM, 6-OHDA-treated DM or control mice were collected, and then the corneal epithelium was scraped and pooled together as a single sample for protein extraction. Each group was collected by three samples. The total protein was extracted from these samples and the lysate was then evenly divided into two aliquots. Following this, the NGF and GDNF were detected by using ELISA kits specific for mouse NGF (USCN Life Science Inc., Wuhan, China) and mouse GDNF (sourced from Abcam) to measure the levels of NGF and GDNF in each aliquot. The concentration of the total protein was measured by the bicinchoninic acid kit (Beyotime, Haimen, China).
RNA extraction and PCR analysis
Total RNA was extracted from the mouse corneal epithelium which was scrapedand collected from corneas and HCECs with a TransZolTM Up Plus RNA kit (TransGen Biotech, Beijing, China) and cDNAs were synthesized using a Hiscript 1st-Strand cDNA Synthesis kit (Vazyme, Nanjing, China). Quantitative PCR (qPCR) was carried out using SYBR Green reagents (Qiagen, Hilden, Germany) and the Roter-Gene Q system (Qiagen, Valencia, Spain) with the specific primers showed in Table S1. The cycling conditions were 2 min at 95 °C followed by 40 two-step cycles (5 s at 95 °C and 10 s at 60 °C).
Statistics and reproducibility
Statistical analysis of biological replicates was performed by Student’s t-test or one-way analysis of variance (ANOVA) with GraphPad Prism 8.0.2 software. The sample sizes and the specific definitions of replicates are detailed in the figure legends. P values are reported in figures as exact values. All experiments were independently repeated at least three times and reproducibility was confirmed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source data behind the graphs in the paper can be found in Supplementary Data 1. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Hu, B. et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020).
Marfurt, C. F. & Ellis, L. C. Immunohistochemical localization of tyrosine hydroxylase in corneal nerves. J. Comp. Neurol. 336, 517–531 (1993).
Conceição, F., Sousa, D. M., Paredes, J. & Lamghari, M. Sympathetic activity in breast cancer and metastasis: partners in crime. Bone Res 9, 9 (2021).
Xue, Y. X. et al. The mouse autonomic nervous system modulates inflammation and epithelial renewal after corneal abrasion through the activation of distinct local macrophages. Mucosal Immunol. 11, 1496–1511 (2018).
Vasamsetti, S. B. et al. Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity 49, 93–106.e7 (2018).
Esler, M. et al. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am. J. Hypertens. 14, 304S–309S (2001).
Seals, D. R. & Bell, C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes 53, 276–284 (2004).
Thorp, A. A. & Schlaich, M. P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 341583 (2015).
Vinik, A. L. & Erbas, T. Recognizing and treating diabetic autonomic neuropathy. Cleve. Clin. J. Med. 68, 928–930 (2001). 932, 934-944.
Freeman, R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 126, 63–79 (2014).
Nishida, T. et al. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J. Cell Physiol. 169, 159–166 (1996).
Yang, L. L. et al. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 63, 4262–4274 (2014).
Garcia-Hirschfeld, J., Lopez-Briones, L. G. & Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp. Eye Res. 59, 597–605 (1994).
Zhang, Y. Y. et al. Role of VIP and sonic hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes 69, 1549–1561 (2020).
Ekstrand, A. J. et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc. Natl. Acad. Sci. USA 100, 6033–6038 (2003).
Mikulec, A. A. & Tanelian, D. L. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J. Ocul. Pharmacol. Ther. 12, 417–423 (1996).
Cai, D., Zhu, M., Petroll, W. M., Koppaka, V. & Robertson, D. M. The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am. J. Pathol. 184, 2662–2670 (2014).
Bonini, S., Lambiase, A., Rama, P., Caprioglio, G. & Aloe, L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 107, 1347–1352 (2000).
Chan, K. Y. & Haschke, R. H. Action of a trophic factor(s) from rabbit corneal epithelial culture on dissociated trigeminal neurons. J. Neurosci. 1, 1155–1162 (1981).
Di, G. H. et al. Corneal epithelium-derived neurotrophic factors promote nerve regeneration. Invest. Ophthalmol. Vis. Sci. 58, 4695–4702 (2017).
Wang, X. C. et al. MANF promotes diabetic corneal epithelial wound healing and nerve regeneration by attenuating hyperglycemia-induced endoplasmic reticulum stress. Diabetes 69, 1264–1278 (2020).
Zhou, Q. J. et al. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells (Dayt., Ohio.) 33, 1566–1576 (2015).
Ezquer, F. E. et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol. Blood Marrow Transpl. 14, 631–640 (2008).
Fong, D. S. et al. Retinopathy in diabetes. Diab. Care 27, S84–S87 (2004).
Lockwood, A., Hope-Ross, M. & Chell, P. Neurotrophic keratopathy and diabetes mellitus. Eye 20, 837–839 (2006).
Callaghan, B. C., Price, R. S., Chen, K. S. & Feldman, E. L. The importance of rare subtypes in diagnosis and treatment of peripheral neuropathy: A review. JAMA Neurol. 72, 1510–1518 (2015).
Dogru, M., Katakami, C. & Inoue, M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 108, 586–592 (2001).
Gao, N. & Yu, F. S. Lack of elevated expression of TGFβ3 contributes to the delay of epithelial wound healing in diabetic corneas. Invest. Ophthalmol. Vis. Sci. 65, 35 (2024).
Zhang, Z. et al. Interference of sympathetic overactivation restores limbal stem/progenitor cells function and accelerates corneal epithelial wound healing in diabetic mice. Biomed. Pharmacother. 161, 114523 (2023).
Di, G. H. et al. VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice. Sci. Rep. 7, 40582 (2017).
Li, F. et al. Autonomic nervous system receptor-mediated regulation of mast cell degranulation modulates the inflammation after corneal epithelial abrasion. Exp. Eye Res. 219, 109065 (2022).
Nakamura, I., Takahashi, C. & Miyagawa, I. The alterations of norepinephrine and acetylcholine concentrations in immature rat urinary bladder caused by streptozotocin-induced diabetes. J. Urol. 148, 423–426 (1992).
Thackeray, J. T. et al. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc. Diabetol. 10, 75 (2011).
Priyadarsini, S. et al. Diabetic keratopathy: insights and challenges. Surv. Ophthalmol. 65, 513–529 (2020).
Babizhayev, M. A. et al. The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem. Biophys. 71, 1425–1443 (2015).
Zhu, L., Titone, R. & Robertson, D. M. The impact of hyperglycemia on the corneal epithelium: Molecular mechanisms and insight. Ocul. Surf. 17, 644–654 (2019).
Zhao, H., He, Y., Ren, Y. R. & Chen, B. H. Corneal alteration and pathogenesis in diabetes mellitus. Int. J. Ophthalmol. 12, 1939–1950 (2019).
Yu, F. S. et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog. Retin. Eye Res. 89, 101039 (2022).
Muller, L. J, Marfurt, C. F, Kruse, F & Tervo, T. M Corneal nerves: Structure, contents and function. Exp. Eye Res. 76, 5542 (2003).
Yu, F. S., Yin, J., Lee, P., Hwang, F. S. & McDermott, M. Sensory nerve regeneration after epithelium wounding in normal and diabetic cornea. Expert Rev. Ophthalmol. 10, 383–392 (2015).
McGettrick, H. M. Bridging the gap-Immune cells that can repair nerves. Cell Mol. Immunol. 18, 784–786 (2021).
Li, Z., Burns, A. R., Han, L., Rumbaut, R. E. & Smith, C. W. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am. J. Pathol. 178, 1106–1116 (2011).
Hoeffel, G. et al. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594, 94–99 (2021).
Leon, A. et al. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 91, 3739–3743 (1994).
Liu, J. et al. Mast cells participate in corneal development in mice. Sci. Rep. 5, 17569 (2015).
Kimura, K. et al. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Auton. Neurosci. 156, 27–35 (2010).
Lambiase, A. et al. NGF topical application in patients with corneal ulcer does not generate circulating NGF antibodies. Pharmacol. Res. 56, 65–69 (2007).
Sacchetti, M. & Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 8, 571–579 (2014).
Lambiase, A., Rama, P., Bonini, S., Caprioglio, G. & Aloe, L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. Surv. Ophthalmol. 338, 1174–1180 (1998).
Pflugfelder, S. C. et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology 127, 14–26 (2020).
Park, J. H., Kang, S. S., Kim, J. Y. & Tchah, H. Nerve growth factor attenuates apoptosis and inflammation in the diabetic cornea. Invest. Ophthalmol. Vis. Sci. 57, 6767–6775 (2016).
Brooks, A. M. V. & Gillies, W. E. Ocular β-blockers in glaucoma management. Drugs Aging 2, 208–221 (1992).
Cinotti, A. et al. Levobunolol vs timolol for open-angle glaucoma and ocular hypertension. Am. J. Ophthalmol. 99, 11–17 (1985).
Sherwood, M. B., Grierson, I., Millar, L. & Hitchings, R. A. Long-term morphologic efects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology 96, 327–335 (1989).
Herreras, J. M., Pastor, J. C., Calonge, M. & Asensio, V. M. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology 99, 1082–1088 (1992).
Kuppens, E. V., Stolwijk, T. R., de Keizer, R. J. & van Best, J. A. Basal tear turnover and topical timolol in glaucoma patients and healthy controls by fluorophotometry. Invest Ophthalmol. Vis. Sci. 33, 3442–3448 (1992).
Niiya, A. et al. Effect of beta-blocker eyedrops on corneal epithelial barrier function. Ophthalmologica 214, 332–336 (2000).
Shiuey, Y. & Eisenberg, M. J. Cardiovascular effects of commonly used ophthalmic medications. ClinCardiol 19, 5–8 (1996).
Yuan, X. Y., Ma, X. B., Yang, L. L., Zhou, Q. J. & Li, Y. β-blocker eye drops affect ocular surface through β2 adrenoceptor of corneal limbal stem cells. BMC Ophthalmol. 21, 419 (2021).
Araki-Sasaki, K. et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 36, 614–621 (1995).
Kawakita, T., Shimmura, S., Hornia, A., Higa, K. & Tseng, S. C. Stratified epithelial sheets engineered from a single adult murine corneal/limbal progenitor cell. J. Cell Mol. Med. 12, 1303–1316 (2008).
Byun, Y. S., Kang, B., Yoo, Y. S. & Joo, C. K. Poly (ADP-ribose) polymerase inhibition improves corneal epithelial innervation and wound healing in diabetic rats. Invest Ophthalmol. Vis. Sci. 56, 1948–1955 (2015).
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (82070927, 82101094 and 81900831), Taishan Scholar Program (tstp20221163, 202211342), the Key Research and Development Program of Shandong Province (2021ZDSYS14), the Natural Science Foundation of Shandong Province (ZR2020QH146, ZR2022MH026 and ZR2022MH042).
Author information
Authors and Affiliations
Contributions
Q.Z. and L.X. conceived and designed the experiments. X.Y., Y.L. and L.C. performed experiments and data analysis. Y.L., X.Y. and Q.Z. writing the paper. L.Y., Y.Z., Z.Z., T.W., M.D. and X.D. assisted with the experimental technical and conducted data analysis. All authors approved the final version of this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
This study has obtained approval from the Ethics Committee of the Eye Institute of Shandong First Medical University (2019-G-015).
Peer review
Peer review information
Communications Biology thanks Laura Calzà, Ting Zhou and Wentao Liang for their contribution to the peer review of this work. Primary Handling Editor: Jasmine Pan. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, X., Li, Y., Cong, L. et al. Norepinephrine regulates epithelial-derived neurotrophins expression and sensory nerve regeneration through ADRB2 receptor. Commun Biol 8, 481 (2025). https://doi.org/10.1038/s42003-025-07903-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07903-5