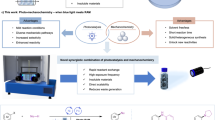
Abstract
Synthetic chemistry approaches for direct C–H bond alkylation offers a promising alternative to traditional functional-group-centered strategies which often involve multi-step procedures and may suffer from a variety of challenges including scalability. Here, we introduce resonant mixing as an efficient method for meta-C–H alkylation of arenes using a Ru-catalyst, avoiding the need for bulk solvents, external temperature, or light. The described methodology is highly rapid, enabling multigram-scale synthesis of meta-alkylation products within a short reaction time and achieving a very high turnover frequency. The reaction operates via a radical mechanism and is characterized by its mild reaction conditions, substrate compatibility, and exceptional meta-selectivity, all while significantly reducing reaction times.

Similar content being viewed by others
Introduction
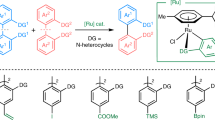
Environmentally benign and sustainable approaches to synthesis are fundamental in contemporary chemical research, as they enable the generation of diverse molecules through greener, more efficient methodologies. Particularly, C–H bond activation and functionalization stand out as a valuable alternative, given the ubiquitous presence of C–H bonds in organic molecules. Consequently, the use of transition metal catalysis for the functionalization of arenes has proven to be a compelling method for accessing heterocycles, pharmaceuticals, and natural products with atom economy and step efficiency, marking significant advancements in the field1,2,3. However, the selective functionalization of differently reactive C–H bonds in arenes introduces complexity to these transformations. To address this issue, various directing groups (DGs) have been elegantly developed to activate the otherwise inert C–H bonds4,5. Notably, activating meta-C–H bonds displays a significant challenge due to the distal position of these bonds, which hampers the formation of stable metallacycles6,7. To overcome these difficulties, different templates, ligand frameworks, and norbornene transient mediators8 have been engineered to precisely position the transition metal (TM) at the meta-C–H site (Fig. 1a)9.
On the other hand, a notable alternative method for meta-C–H functionalization of arenes has been developed using a Ru-catalyst that undergoes ortho-cycloruthenation, thereby exerting a para-directing effect (Fig. 1b)10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30. Leveraging this strategy, Ackermann and his team achieved Ru-catalyzed meta-alkylation using secondary alkyl halides29, an approach subsequently broadened to encompass tertiary alkyl halides26,27. These results mark an important advancement in the area of arene distal-C–H functionalization. Lately, a notable visible light-mediated Ru-catalyzed meta-C–H alkylation has been developed by Ackermann14,18 and Greaney19, independently using alkyl bromides and iodides under ambient conditions. These reactions hinge on the formation of an ortho-cycloruthenation intermediate, which enables single electron transfer (SET) with alkyl halides on photo-irradiation. Despite significant advancements in meta-C–H functionalization, the majority of these methods often require prolonged reaction times and depend on external heating/light irradiation. Furthermore, scalability remains a significant challenge that requires attention.
Given the importance of distal C–H functionalization, we chose to explore and establish an efficient, rapid, and scalable method based on resonant mixing to selectively alkylate the meta-C–H bond in arenes (Fig. 1c). To the best of our knowledge, no prior work has been reported on mechanochemical or resonant mixing accelerated meta-C–H functionalization, although the method could be exceptionally rapid and economically favorable. Resonant acoustic mixing (RAM) is a non-contact, vibrational technique that utilizes low-frequency energy to create rapid mechanical agitation. This method produces a unique reaction movement facilitating rapid and uniform mixing. It achieves this by adjusting the vertical acceleration of the reaction vessel and thus eliminates the need for milling or grinding media, thereby preventing product contamination due to abrasion. This simplifies reaction design and facilitates scale-up31. RAM has been gaining room as a technique used in different production processes, including formulations, coatings, composite and nanomaterial preparation, co-crystallizations, mixing of high-energy materials, and more recently in organic synthesis31,32,33,34,35,36,37,38,39,40,41,42,43.
Results and discussion
Recognizing the significant impact of mechanochemistry, recently highlighted by IUPAC as a transformative chemical technology44, and the benefits of resonant mixing, we initiated an investigation into Ru-catalyzed meta-C–H alkylation.
Reaction optimization
We started the optimization by reacting 2-phenylpyridine (1a, 0.1 mmol) and tert-butyl iodide (2a, 0.3 mmol) with [RuCl2(p-cymene)]2 (10 mol%), KOAc (50 mol%), PPh3 (20 mol%), and K2CO3 (1.5 equiv.) in 1,4-dioxane (400 μL) using the resonant mixer at 90 g (g = 9.81 m s-2) vertical acceleration for 1-2 h. We were delighted that the meta-alkylation product 3a obtained in 7–16% yield (Table 1, entry 1, see supplementary information for further details). Interestingly, carrying the reaction in 0.4 mmol scale with 5 mol% of Ru, and 10 mol% of PPh3 increased the yield to 46%, whereas the reaction with 2.5 mol% and 10 mol% Ru gave the product in 41% and 52% yield, respectively (entry 2). Increasing the reaction time to 3 h did not have a significant effect (49%, entry 2). However, minimal solvent assistance is crucial as the reaction without solvent gave a lower yield of 37% compared to 52% with solvent (entry 3). We tested the reaction with varying amounts of alkyl halide, where 3 equiv. of alkyl iodide was found to be better (52%, see SI). In comparison, using 1 and 2 equiv. of alkyl iodide resulted in yields of 28% and 41%, respectively. The use of additives such as sand and glass beads improved the reactivity and the use of sand in the presence of 200 μL solvent at η = 0.33 µL mg−1resulted in the formation of the product in 84% yield (entries 4 and 5). The use of other bases such as KHCO3 and K3PO4 or decreasing the loading of K2CO3 had a detrimental effect on the reaction yield (entries 6-9). Increasing the loading of K2CO3 showed an upsurge in yield at η = 0.32 µL mg−1 (94% in 2 h), and no exogenous grinding reagent is required (entry 10). Interestingly, at η = 0.64 µL mg−1 nearly complete conversion of the starting material was observed with a yield of the product 98% in 2 h and 94% in 1 h (entry 11). The impact of the resonant acceleration on the reaction yield was tested which showed that the reaction at 90 g is optimal (entry 12). In the absence of PPh3, the yield decreased to 33% signifying the crucial role of phosphine in the catalytic process (entry 13). Interestingly, alkyl bromides that are less reactive compared to alkyl iodides also gave a reproducible yield of 97% at η = 0.7 µL mg−1 under identical conditions; however, a slightly longer reaction time is required (entries 14 and 15, see SI for full details).
Scope of substrates
With the optimized reaction condition, we investigated the scope of Ru-catalyzed meta-alkylation by employing various arenes and alkyl halides (Fig. 2). We first tested various heteroarenes 1 with tert-butyl bromides and iodides 2, which gave the desired meta-C–C cross-coupled product 3a-3v in good to excellent yields establishing 2-phenylpyridine as an effective substrate for selective meta-alkylation in resonant mixing. The literature emphasizes the importance of either thermal energy (∼100 °C) or light irradiation to drive these reactions, often requiring extended reaction times. Particularly, the reaction with alkyl bromides is notably challenging even at 80 °C in the absence of light14,18. Nevertheless, under the resonant conditions employed in the present study, both the alkyl bromides and iodides were found equally efficient giving excellent yields of meta-alkylation. Generally, solution-based reactions of pyrazole substrates with tert-butyl bromides for meta-C–H alkylation are rather difficult, typically necessitating both thermal heating and photo-irradiation, often resulting in lower yields18. The same is the case with oxazoline substrates that exhibit diminished reactivity in solution under both the photoredox (31%, 48 h), and thermal conditions (37%, 24 h)14,18. However, heteroarenes such as pyrazole (3j and 3k, up to 81%), oxazoline (3l, up to 84%), and benzo[h]quinoline (3m) were found to be efficient directing groups for meta-C−H alkylation under the resonant conditions utilized. Notably, the pyrimidine substrate was effectively reacted using resonant mixing, yielding meta-alkylation products 3h and 3i in excellent yields of up to 96%. The viability of the reaction was further tested with 4-bromoheptane as the coupling partner which gave the desired meta-alkylation product on 2-phenyl pyridine (3n), substituted pyridines (3o-3s), pyrimidine (3t and 3u), and pyrazole (3v) in good to moderate yields. The meta-alkylation reaction of pyrimidine with secondary alkyl bromide typically yields a mixture of mono- and di-meta-alkylated products in solution18. In contrast, the current RAM methodology exhibited excellent selectivity, yielding only the mono-meta-alkylated products (3t and 3u). Next, we focused on broadening the scope by employing various secondary and tertiary alkyl bromides, which also afforded the anticipated meta-C(sp2)–C(sp3) cross-coupled product in good to moderate yields. Unactivated acyclic, and cyclic secondary alkyl bromides reacted well under the reaction conditions, furnishing the alkylation products 3w-3aa in moderate yields of 48-60%. Sterically hindered bridged bromobicyclo[2.2.1]heptane (3ab) was also tolerated, however, a diminished reactivity was observed. In comparison to secondary alkyl halides, tertiary alkyl bromides (3ac-3ae) exhibited enhanced reactivity due to their increased radical stability. Unlike the alkyl halides discussed above, alkyl bromides containing withdrawing groups produce electrophilic radicals that are less reactive in solution, requiring extended reaction times with continuous irradiation or heating18,27. Interestingly, resonant mixing was equally effective in selectively incorporating alkyl bromides with withdrawing groups onto the meta-position 3af with a relatively shorter reaction time. As expected, primary alkyl halides yielded ortho-alkylation products (3ag-3aj) likely due to the two-electron oxidative addition of alkyl halide to Ru rather than the SET45,46. However, these reactions in solution typically require elevated reaction temperatures45 or light irradiation with a long reaction time19.
Yields of isolated products. Reaction time of 2 h for alkyl iodides, and 3 h for alkyl bromides. aRegioisomeric mixture (m:o = 5:1) determined by 1H NMR analysis (Supplementary Data 1).
To further establish the generality of the present protocol, the reaction between 2-phenylpyridine (1a) and ethyl 2-bromo-2,2-difluoroacetate (4) was performed under slightly modified reaction condition with [RuCl2(p-cymene)]2 which produced the meta-difluoroalkylation product 5 in 51% in 1 h, with a turnover frequency (TOF) of 2.6 h−1 (Fig. 3a). It is worth noting that meta-C−H difluoroalkylation has been achieved previously in solution using photochemical methods (TOF = 0.1 h−1)18, and under conventional heating (TOF = 0.2 h−1)10,22. However, the photochemical approach typically requires longer reaction times, up to 48 h, whereas conventional heating requires a preformed Ru-complex [Ru(O2CMes)2(p-cymene)] along with phosphine or nickel additives, while RuCl2(p-cymene)]2 was found to be completely inactive for this transformation. Alternatively, Wang and coworkers employed a palladium co-catalyst in addition to [RuCl2(p-cymene)]2 to activate the difluoroalkyl bromide, where only a trace product was obtained without the Pd co-catalyst47,48. Relatively, the developed methodology utilizing resonant mixing is notably efficient, featuring a high turnover frequency, and does not require a preformed Ru-complex and co-catalyst.
Next, scale-up reactions were carried out to unveil the wider applicability of the present protocol. Initially, a gram scale reaction of 2-phenylpyridine (1a, 6.44 mmol, 1.0 g) was performed under standard conditions at resonant mixing of 90 g for 2 h, yielding the meta-alkylation product 3a in 91% (1.23 g, Fig. 3b, see SI for further details). Interestingly, no drop in yield was observed in the gram scale synthesis, achieving a higher turnover frequency of 4.6 h−1. Subsequently, we investigated a multi-gram scale synthesis (160-fold scale-up) with reduced loading of Ru-catalyst (1.0 mol%), ligand (2.0 mol%), KOAc (10 mol%), and shortened reaction time (30 min). Remarkably, the lower catalyst loading also furnished the meta-alkylation product 3a in 84% yield (11.41 g), with an excellent TOF of 84 h−1 (Fig. 3c). Further lowering the loading of Ru-catalyst (0.5 mol%), ligand (1.0 mol%), band KOAc (5 mol%), also showed promising scalability producing 3a in 73% yield with a TOF of 146 h−1 (Fig. 3c).
Mechanistic investigation
Next, we turned our attention to control and competition experiments to gain insight into the reaction mechanism. To investigate the involvement of SET in the reaction mechanism, the radical scavenger TEMPO was used under the standard condition, which completely suppressed the product formation indicating a radical process (Fig. 4a)18. To further confirm this, other radical scavengers such as butylated hydroxytoluene (BHT), and 1,1-diphenylethylene were used which also suppressed the reaction affirming the radical process (Fig. 4a). Succeeding, intermolecular competition experiments by varying the arene and alkyl coupling partners were carried out, where the reaction between –OMe (1d) and –CF3 (1e) substituted arenes displayed preferential meta-alkylation on methoxy-substituted arene suggesting an electrophilic ruthenation (Fig. 4b)29. Further, a competition experiment between tertiary 2b and secondary 2c alkyl bromides showed a twofold increase in reactivity for the tertiary alkyl bromide (Fig. 4c). Following, meta-alkylation with isotopically labeled arene [D5]-1a displayed a significant D/H exchange at the ortho-position of the alkylated product [Dn]-3a, suggesting that the ortho-ruthenation is reversible (Fig. 4d, see SI for further details). Interestingly, D/H exchange under resonant mixing occurs at a slower rate compared to the solution-based reaction under thermal conditions26. This implies that the ortho-ruthenation intermediate B is more reactive under the RAM conditions, favoring the forward reaction. A similar reaction using MeOD under the standard condition also presented H/D exchange at the ortho-position of the product 3a’. However, this exchange was not significant, confirming that the reversible C–H ruthenation proceeds at a slower rate (Fig. 4e). Drawing on these mechanistic insights and previous studies, we propose a mechanism for Ru-catalyzed meta-alkylation, as illustrated in Fig. 5. Our control experiments indicate that both KOAc and PPh3 are indispensable. A similar observation was also made by Ackermann and co-workers, where [RuCl2(p-cymene)]2 was found completely inactive for their meta-alkylation, while [Ru(OAc)2(p-cymene)] in combination with phosphine additive were found active22. These findings suggest that phosphine and carboxylate synergistically facilitate the formation of the catalytically active Ru-complex a. This complex undergoes reversible ortho-C–H ruthenation to form intermediate b, as verified by D/H and H/D exchange studies. The application of rapid mechanical agitation at 90 g likely promotes a more homogeneous reaction environment, enhancing collision efficiency and leading to the generation of an alkyl radical via single-electron transfer from Ru to the alkyl halide. This nucleophilic alkyl radical subsequently attacks the para-position of the electrophilic complex c, forming a radical cation intermediate d. Deprotonation and rearomatization in the presence of a base then produce the meta-alkylated intermediate e. Finally, protodemetalation of complex e in the presence of KHCO3 and KOAc yields the meta-alkylated product 3, simultaneously regenerating the catalytically active ruthenium(II) species a49.
Conclusion
In summary, we have developed a rapid and scalable meta-alkylation methodology that expedites the reaction of heteroarenes with alkyl halides using a ruthenium catalyst. This process employs resonant acoustic mixing to homogenize the reaction, promoting efficient molecular collisions, thus avoiding the need for external heating or a light source, and reducing solvent usage. This approach is compatible with a variety of secondary and tertiary alkyl bromides and iodides, yielding products with high selectivity and excellent turnover frequencies up to 146 h−1. The proposed mechanism is supported by isotope labeling and competition experiments and involves the formation of a nucleophilic alkyl radical. These results demonstrate the potential of resonant mixing to substantially advance challenging catalytic reactions, making them faster, more efficient, and scalable.
Methods
Detailed reagent purifications and specific experimental procedures for individual compounds are described in the Supplementary Methods.
General procedure for Ru-catalyzed meta-alkylation by RAM
A clean 2 mL screw cap glass reaction vial was charged with [Ru(p-cymene)Cl2]2 (12.2 mg, 0.02 mmol, 5 mol%), PPh3 (10.5 mg, 0.04 mmol, 10 mol%), and heteroarene (1.0 equiv., 0.4 mmol). To that, granular K2CO3 (300 mg, 2.17 mmol, 5.43 equiv.), KOAc (19.6 mg, 0.2 mmol, 50 mol%), and degassed 1,4-dioxane (400 μL) were added followed by alkyl halide (1.2 mmol, 3.0 equiv). The vial, capped with Teflon septum, was placed in the LabRAM II instrument for 2–3 h (2 h for alkyl iodide, 3 h for alkyl bromide) at an acoustic acceleration of 90 g. Upon completion, the reaction was exposed to air. The reaction mixture was diluted with EtOAc, filtered through celite, and concentrated in vacuo. The residue was purified by column chromatography. All the compounds were fully characterized (see Supplementary Information).
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files. The experimental procedures, detailed optimization conditions, product characterization of all new compounds are provided in the “Supplementary Information” file. “Supplementary Data 1” contains all 1H and 13C NMR spectra. All data are available from the corresponding author upon request.
References
Sinha, S. K. et al. Transition-metal catalyzed C–H activation as a means of synthesizing complex natural products. Chem. Soc. Rev. 52, 7461–7503 (2023).
Rogge, T. et al. C–H activation. Nat. Rev. Methods Prim. 1, 43 (2021).
Lam, N. Y. S., Wu, K. & Yu, J.-Q. Advancing the logic of chemical synthesis: C−H activation as strategic and tactical disconnections for C−C bond construction. Angew. Chem. Int. Ed. 60, 15767–15790 (2021).
Rej, S., Ano, Y. & Chatani, N. Bidentate directing groups: An efficient tool in C–H bond functionalization chemistry for the expedient construction of C–C bonds. Chem. Rev. 120, 1788–1887 (2020).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Sinha, S. K. et al. Toolbox for distal C–H bond functionalizations in organic molecules. Chem. Rev. 122, 5682–5841 (2022).
Lam, N. Y. S. et al. Empirical guidelines for the development of remote directing templates through quantitative and experimental analyses. J. Am. Chem. Soc. 144, 2793–2803 (2022).
Gandeepan, P. & Ackermann, L. Transient directing groups for transformative C–H activation by synergistic metal catalysis. Chem 4, 199–222 (2018).
Meng, G. et al. Achieving site-selectivity for C–H activation processes based on distance and geometry: A carpenter’s approach. J. Am. Chem. Soc. 142, 10571–10591 (2020).
Guillemard, L., Ackermann, L. & Johansson, M. J. Late-stage meta-C–H alkylation of pharmaceuticals to modulate biological properties and expedite molecular optimisation in a single step. Nat. Commun. 15, 3349 (2024).
Luan, Y.-Y. et al. Ruthenium-catalyzed difunctionalization of vinyl cyclopropanes for double m-C(sp2)–H/C-5(sp3)–H functionalization. Org. Lett. 26, 3213–3217 (2024).
Luo, X. et al. Ligand-enabled ruthenium-catalyzed meta-C−H alkylation of (hetero)aromatic carboxylic acids. Nat. Commun. 15, 5552 (2024).
Chen, S., Yuan, B., Wang, Y. & Ackermann, L. Ruthenium-catalyzed remote difunctionalization of nonactivated alkenes for double meta-C(sp2)−H/C-6(sp3)−H functionalization. Angew. Chem. Int. Ed. 62, e202301168 (2023).
Wang, Y., Chen, S., Chen, X., Zangarelli, A. & Ackermann, L. Photo-induced ruthenium-catalyzed double remote C(sp2)−H/C(sp3)−H functionalizations by radical relay. Angew. Chem. Int. Ed. 61, e202205562 (2022).
Gou, X.-Y. et al. Ruthenium-catalyzed radical cyclization/meta-selective C–H alkylation of arenes via σ-activation strategy. ACS Catal. 11, 4263–4270 (2021).
Korvorapun, K., Kuniyil, R. & Ackermann, L. Late-stage diversification by selectivity switch in meta-C–H Activation: Evidence for Singlet Stabilization. ACS Catal. 10, 435–440 (2020).
Shi, W.-Y. et al. Three-component ruthenium-catalyzed remote C–H functionalization of 8-aminoquinoline amides. Chem. Commun. 56, 12729–12732 (2020).
Gandeepan, P., Koeller, J., Korvorapun, K., Mohr, J. & Ackermann, L. Visible-light-enabled ruthenium-catalyzed meta-C−H alkylation at room temperature. Angew. Chem. Int. Ed. 58, 9820–9825 (2019).
Sagadevan, A. & Greaney, M. F. meta-Selective C−H activation of arenes at room temperature using visible light: dual-function ruthenium catalysis. Angew. Chem. Int. Ed. 58, 9826–9830 (2019).
Khan, F. F., Sinha, S. K., Lahiri, G. K. & Maiti, D. Ruthenium-mediated distal C−H activation. Chem. Asian J. 13, 2243–2256 (2018).
Paterson, A. J. et al. α-Halo carbonyls enable meta-selective primary, secondary and tertiary C–H alkylations by ruthenium catalysis. Org. Biomol. Chem. 15, 5993–6000 (2017).
Ruan, Z. et al. Ruthenium(II)-catalyzed meta C−H mono- and difluoromethylations by phosphine/carboxylate cooperation. Angew. Chem. Int. Ed. 56, 2045–2049 (2017).
Li, J. et al. Ruthenium(II)-catalysed remote C–H alkylations as a versatile platform to meta-decorated arenes. Nat. Commun. 8, 15430 (2017).
Leitch, J. A., McMullin, C. L., Mahon, M. F., Bhonoah, Y. & Frost, C. G. Remote C6-selective ruthenium-catalyzed C–H alkylation of indole derivatives via σ-activation. ACS Catal. 7, 2616–2623 (2017).
Leitch, J. A. & Frost, C. G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalisation. Chem. Soc. Rev. 46, 7145–7153 (2017).
Li, J. et al. N-Acyl amino acid ligands for ruthenium(II)-catalyzed meta-C–H tert-alkylation with removable auxiliaries. J. Am. Chem. Soc. 137, 13894–13901 (2015).
Paterson, A. J., St John-Campbell, S., Mahon, M. F., Press, N. J. & Frost, C. G. Catalytic meta-selective C–H functionalization to construct quaternary carbon centres. Chem. Commun. 51, 12807–12810 (2015).
Yu, Q., Hu, L. A., Wang, Y., Zheng, S. & Huang, J. Directed meta-selective bromination of arenes with ruthenium catalysts. Angew. Chem. Int. Ed. 54, 15284–15288 (2015).
Hofmann, N. & Ackermann, L. meta-Selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 135, 5877–5884 (2013).
Saidi, O. et al. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011).
Nanni, A., Kong, D., Zhu, C. & Rueping, M. Nickel-catalyzed cross-coupling aminations via high-throughput mechanochemistry enabled by resonant acoustic mixing. Green. Chem. 26, 8341–8347 (2024).
Hu, C. et al. A new approach for preparing stable high-concentration peptide nanoparticle formulations. Pharmaceuticals 17, 15 (2024).
Tariq, Q. et al. Comparative studies of synthesis, performance, and applications of recently developed CL-20 based co-crystals. Cryst. Growth Des. 23, 6974–6987 (2023).
Wohlgemuth, M. et al. Milling medium-free suzuki coupling by direct mechanocatalysis: From mixer mills to resonant acoustic mixers. Chem. Eur. J. 29, e202301714 (2023).
Effaty, F. et al. Resonant acoustic mixing (RAM) for efficient mechanoredox catalysis without grinding or impact media. Chem. Commun. 59, 1010–1013 (2023).
Gonnet, L. et al. The “η-sweet-spot” (ηmax) in liquid-assisted mechanochemistry: polymorph control and the role of a liquid additive as either a catalyst or an inhibitor in resonant acoustic mixing (RAM). Faraday Discuss 241, 128–149 (2023).
Lennox, C. B. et al. Direct mechanocatalysis by resonant acoustic mixing (RAM). Chem. Sci. 14, 7475–7481 (2023).
Gonnet, L. et al. Metal-catalyzed organic reactions by resonant acoustic mixing. Angew. Chem. Int. Ed. 61, e202115030 (2022).
Wright, C. J. et al. Is ResonantAcoustic Mixing®(RAM) a game changer for manufacturing solid composite rocket propellants? Propellants Explos. Pyrotech. 47, e202100146 (2022).
Vandenberg, A. & Wille, K. Evaluation of resonance acoustic mixing technology using ultra high performance concrete. Constr. Build. Mater. 164, 716–730 (2018).
Osorio, J. G., Hernández, E., Romañach, R. J. & Muzzio, F. J. Characterization of resonant acoustic mixing using near-infrared chemical imaging. Powder Technol. 297, 349–356 (2016).
am Ende, D. J., Anderson, S. R. & Salan, J. S. Development and scale-up of cocrystals using resonant acoustic mixing. Org. Proc. Res. Dev. 18, 331–341 (2014).
Makarov, A. S. & Rueping, M. Scalable depolymerizing transesterification and amidation of (poly)lactic acid (PLA) enabled by resonant acoustic mixing (RAM). Green Chem. 27 (2025).
Gomollón-Bel, F. & García-Martínez, J. Emerging chemistry technologies for a better world. Nat. Chem. 14, 113–114 (2022).
Ackermann, L., Hofmann, N. & Vicente, R. Carboxylate-assisted ruthenium-catalyzed direct alkylations of ketimines. Org. Lett. 13, 1875–1877 (2011).
Ackermann, L., Novák, P., Vicente, R. & Hofmann, N. Ruthenium-catalyzed regioselective direct alkylation of arenes with unactivated alkyl halides through C–H bond cleavage. Angew. Chem. Int. Ed. 48, 6045–6048 (2009).
Li, Z.-Y. et al. Ruthenium-catalyzed meta-selective C−H mono- and difluoromethylation of arenes through ortho-metalation strategy. Chem. Eur. J. 23, 3285–3290 (2017).
Cui, P.-C. & Wang, G.-W. Visible-light-mediated bimetal-catalyzed meta-alkylation of arenes. Org. Lett. 26, 427–432 (2024).
Chen, X. et al. Close‐shell reductive elimination versus open‐shell radical coupling for site‐selective ruthenium‐catalyzed C− H activations by computation and experiments. Angew. Chem. Int. Ed. 62, e202302021 (2023).
Acknowledgements
This publication is based upon work supported by the King Abdullah University of Science and Technology (KAUST), Office of Sponsored Research (OSR) under CRG award no. URF/1/4405.
Author information
Authors and Affiliations
Contributions
M.R., R.K. and A.D. conceived and designed the project. A.D., R.K., K.P. and N.K. performed and analyzed the experiments. M.R., A.D., and R.K. wrote the manuscript with input from others. A.D., R.K., and K. P. prepared the supplementary information. M.R. directed the whole research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dey, A., Kancherla, R., Pal, K. et al. Rapid and scalable ruthenium catalyzed meta-C–H alkylation enabled by resonant acoustic mixing. Commun Chem 7, 295 (2024). https://doi.org/10.1038/s42004-024-01390-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-024-01390-1
This article is cited by
-
A scalable photo-mechanochemical platform for sustainable photoredox catalysis by resonant acoustic mixing
Nature Communications (2025)