Abstract
Psoralen-conjugated triplex-forming oligonucleotides (Ps-TFOs) have been employed for the photodynamic regulation of gene expression by the photo-cross-linking of psoralen with the target DNA. However, stable triplex formation requires a consecutive purine base sequence in one strand of the target DNA duplexes. The pyrimidine-base interruption in the consecutive purine base sequence drastically decreases the thermodynamic stability of the corresponding triplex, which hampers the TFO application. Here, we propose a design of the Ps-TFO for stable triplex formation with target DNA sequences containing pyrimidine-base interruptions under physiological conditions. This Ps-TFO, named 1’(one)-psoralen-conjugated triplex-forming oligonucleotide (OPTO), incorporates a synthesized nucleoside mimic 1’-psoralen-conjugated deoxyribose to increase the thermodynamic stability of the corresponding triplex by the intercalation of psoralen. The triplex-forming abilities of the OPTO were successfully demonstrated in combination with LNA and 5-methylcytosine, indicating that the use of OPTO will expand the range of the target sequences of TFO for photodynamic gene regulation.
Similar content being viewed by others
Introduction
A triplex-forming oligonucleotide (TFO) binds to its target DNA duplex, forming a triple-helix structure. This triplex formation inhibits either the binding of transcription factors to promoter regions or transcriptional elongation, resulting in gene suppression1,2,3,4. Moreover, the cell recognizes the triplex structure as unusual, which induces a double-strand break (DSB) at the triplex-forming site by an endogenous nuclease5. This DSB induction by a TFO has been employed for genome editing or selectively inducing apoptosis in cancer cells6,7,8,9,10. Recently, we also describe the advantages of using TFO for genome targeting tools in comparison to protein-based genome editing tools such as ZFNs, TALENs, and CRISPR-Cas9. These protein-based tools are suffering from their delivery and off-target gene manipulation. Moreover, the use of exogenous nuclease causes several drawbacks such as cost and immune response. In this point, nucleic acid-based approaches such as TFO have several advantages in terms of the problems mentioned above11.
The interaction of a TFO composed of pyrimidine bases with the target DNA occurs on the major groove of a DNA duplex via Hoogsteen hydrogen bonds (parallel triplex; Fig. 1a). The inherent challenge of the triplex technology is the requirement for Hoogsteen hydrogen bonds to form specifically between a purine base of A:T (or G:C) base pairs in the double-stranded DNA (dsDNA) and a TFO. If the target base-pair in the dsDNA changes (T:A or C:G base-pair), a mismatched base-pair (pyrimidine-base-interrupting site) with the TFO is produced, significantly reducing the thermodynamic stability of the triplex structure (Fig. 1b). In the parallel motif, the N3 position of cytosine must be protonated to facilitate hydrogen bonding, which further makes it difficult to form parallel triplex in physiological pH conditions.
Researchers have explored various modifications to the sugar, phosphate, and base moieties of TFOs to address the primary challenges of pH-dependent thermodynamic stability and the need for polypurine sequences in target genes for parallel triplexes12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27. Notably, Brown et al. incorporated a thiazole orange (TO) intercalator into the thymine nucleobase of parallel TFOs26. This modification significantly increased the melting temperature (Tm) of a parallel triplex containing a TFO with three TO units and a target duplex with a single pyrimidine-base-interrupting site, even under neutral conditions (pH 7.0). Such an approach can broaden the scope of target duplexes accessible under physiological conditions; thus, it warrants further exploration, alongside other gene-directed control strategies using TFOs. Among DNA intercalators, we focus on psoralen, a well-known DNA photo-cross-linking agent. Psoralen derivatives react with pyrimidine bases through a [2 + 2] photo-cycloaddition reaction upon photo-irradiation, forming a cyclobutane ring to give monoadduct or diadduct products28. The cross-linking ability of psoralen has been employed to enhance and control the biological activities of TFOs6,29,30,31,32,33,34,35,36,37,38. Recently, our group demonstrated the inhibition of endogenous gene expression using a TFO equipped with a psoralen moiety at its 5’-end (5’-Ps-TFO) (Fig. 2). This illustrated that UV (365 nm) irradiation induces the cross-linking of a 5’-Ps-TFO to its target DNA, thereby significantly reducing the target gene expression38. Therefore, employing psoralen for triplex stabilization is expected to enhance the biological efficacy of TFOs. One report described the photo-cross-linking of psoralen with the internal pyrimidine base of target DNA duplexes by introducing psoralen into the C-5 position of thymine. In this report, the photo-cross-linking of psoralen improved the thermodynamic stability of the corresponding triplex dramatically. However, it was only demonstrated at acidic conditions with only one sequence. In our opinion, the stable triplex formation is a prerequisite factor for efficient photo-cross-linking at physiological condition39.
In this study, we design a compound with psoralen positioned at the C-1 position of deoxyribose (1’-psoralen-conjugated deoxyribose, P). We introduce it into the mismatch base-pair formation site in the TFO (Fig. 2). This proposed TFO, termed 1’(one)-psoralen-conjugated triplex-forming oligonucleotide (OPTO), incorporates a psoralen moiety to enhance its intercalation into the target DNA. We hypothesize that introducing P into the mismatch site of TFO can improve the thermodynamic stability of the triplex through intercalation, and psoralen can form interstrand cross-linking at the mismatch site. As the target sequence of OPTO, we select a partial sequence from the 5’-long terminal repeats (LTRs) of the human T-cell leukemia virus type 1 (HTLV-1) genome. The 5’-LTR contains critical elements for HTLV-1 replication, including promoter and enhancer sequences. HTLV-1 is responsible for adult T-cell leukemia, HTLV-1-associated myelopathy, and neurological disorders40,41,42. Considering the latent nature of the virus, eliminating this viral sequence from the host genome is crucial for achieving a cure for these diseases. However, current therapeutic approaches show limited efficacy43,44,45. CRISPR-Cas9-based approach has been considered recently, however, there are many hurdles to overcome, such as delivery and off-target effects like those mentioned above, before it can be clinically available46. If TFOs can form stable triplexes with the 5′-LTR of the HTLV-1 provirus genome, they can serve as potent candidates for the radical treatment of these diseases45 through the further engineering of TFO11, using the TFO as a scaffold. However, the polypurine target (PPT) sequence in the 5′-LTR region of HTLV-147 contains at least two mismatched base pairs (Supplementary Fig. S1), posing a challenge for conventional TFOs to form stable triplexes. Therefore, these sequences are ideal targets for demonstrating the efficacy of the OPTO method. Here, the OPTO formed a more stable triplex with this target sequence than conventional TFO in combination with locked nucleic acid (LNA)12,48,49,50,51 and 5-methylcytosine (5mC)52. Cross-linking of OPTO to the target DNA duplex upon UV irradiation was also demonstrated. These results indicate that the use of the OPTO will expand the range of the target sequences of TFO for photodynamic gene regulation.
Results and discussion
Synthesis and functional evaluation of the 1’(one)-psoralen-conjugated triplex-forming oligonucleotide
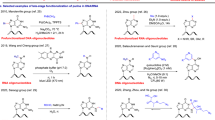
The synthesis of P began with the introduction of a cyano group into Hoffer’s chlorosugar (1), following a procedure described in the literature53 (Scheme 1). The treatment of 1 with BF3·OEt2 and cyanotrimethylsilane in CH2Cl2 afforded 2 in 58% yield. Compound 2 was prepared using a reported procedure54, with slight modifications. The subsequent treatment of 2 with sodium methoxide in MeOH/H2O led to nitrile hydrolysis and in situ esterification, consequently removing the toluoyl group and forming 3 in 68% yield. The two hydroxyl groups of ester 3 were protected with tert-butyldimethylsilyl (TBS) to produce 4 in 71% yield, followed by the hydrolysis of ester 4 to obtain carboxylic acid 5 in 98% yield. It was hypothesized that a C5 linker would be suitable for psoralen intercalation (Supplementary Fig. S2). Thus, linker 6 was introduced through amide condensation to produce 7 in 76% yield. Additionally, a C4 linker version of this compound was synthesized (Supplementary Methods). The primary alcohol of amide 7 was activated by converting it into a methanesulfonic acid ester (8) in 87% yield. The nucleophilic substitution of the Ms group in 8 with psoralen yielded amide 9 in 96% yield. The deprotection of the two TBS groups in 9 using tetrabutylammonium fluoride (TBAF) afforded 10 in 70% yield. For the solid-phase synthesis of OPTO, the primary alcohol of 10 was protected with a 4,4′-dimethoxytrityl group to produce 11 in 86% yield. Finally, the reaction of 11 with 1H-tetrazole and 2-cyanoethyl N,N,Nʹ,Nʹ-tetraisopropylphosphordiamidite in CH2Cl2 afforded phosphoramidite (12) in 87% yield. Subsequently, 12 was employed in the solid-phase synthesis of the OPTO conducted at Ajinomoto Genedesign (Osaka, Japan), as detailed in the Supplementary method. Thereafter, the functional evaluations of the OPTO were performed. Based on the G content ratio and location, we chose a target duplex (606-Py/606-Pu) in the promoter region as the PPT. The sequences of the PPT duplex (606-Py/606-Pu), normal TFO, and OPTO are illustrated in Fig. 3a. The OPTO incorporated P (Fig. 3b) at the mismatch sites of the duplex, whereas normal TFO had a thymine base. The Tm values of each triplex (duplex/TFO) and (duplex/OPTO), were determined from UV-melting profiles at pH 5.3 (Fig. 3c), and a biphasic melting profile with distinct first and second transitions was revealed. The first transition represented the triplex, and the Tm values for the triplexes were 17 °C (duplex/TFO) and 21 °C (duplex/OPTO). Introducing P enhanced the thermodynamic stability of the triplex by 4 °C, and the linker length (C4 or C5) of P did not significantly affect the stability of the triplex (Supplementary Fig. S3). Therefore, the C5 linker was selected for subsequent experiments.
Synthesis of 1’-psoralen-conjugated deoxyribose phosphoramidite (12). Reagents and conditions: (i) trimethylsilyl cyanide (1.5 equiv), BF3·Et2O (0.3 equiv), CH2Cl2, RT, N2, 1 d, 58% yield; (ii) 1. 28% NaOMe solution in Methanol (6 equiv), H2O (3 equiv), Methanol, RT, 4 h; 2. strong acidic cation exchange resin No. 4 (8% cross-linking, 50-100 mesh, H type, 21 g), RT, 1 h, 68% yield; (iii) TBSCl (3 equiv), imidazole (3 equiv), CH2Cl2, RT, N2, overnight, 71% yield; (iv) 1 M NaOH solution (1 equiv), MeOH/THF (1:1), RT, N2, 0.5 h, 98% yield; (v) 5-aminopentanol (2 equiv), HBTU (2 equiv), triethylamine (4 equiv), CH2Cl2, RT, N2, overnight, 76% yield; (vi) methanesulfonyl chloride (1.2 equiv), pyridine, RT, N2, 3 h, 87% yield; (vii) 5-hydroxypsoralen (1.2 equiv), potassium carbonate (1.2 equiv), DMF, 60 ˚C, N2, 3 h, 96% yield; (viii) 1 M solution of tetrabutylammonium fluoride in THF (2.4 equiv), THF, 60 ˚C, N2, 2 h, 70% yield; (ix) 4, 4ʹ-dimethoxytrityl chloride (1.1 equiv), pyridine, RT, N2, 1.5 h, 86% yield; (x) 1H-tetrazole (1.2 equiv), 2-cyanoethyl N,N,N’,N’-tetraisopropylphosphordiamidite (1.2 equiv), CH2Cl2, RT, N2, 1.5 h, 87% yield.
a sequences of the polypurine target (PPT) duplex (606-Py/606-Pu), TFO, and OPTO. b Structure of the 1’-psoralen-conjugated deoxyribose (P). c Normalized UV-melting curves of triplexes (duplex/TFO) and (duplex/OPTO) at pH 5.3. The final concentration of duplex and TFO was 3 μM each. The melting temperature (Tm) values are displayed as the mean ± standard deviation (s.d.) for n = 3 replicates.
Synergistic effect of the locked nucleic acid and 1’(one)-psoralen-conjugated triplex-forming oligonucleotide on triplex stability
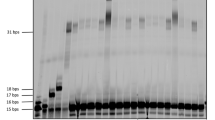
Considering physiological conditions (37 °C, pH 7.0), the previously described Tm value of OPTO was insufficient. To enhance the thermodynamic stability of the triplex at the same PPT site, we explored a combinatorial approach using P along with other artificial nucleotides. In our previous work, we used LNA12,48,49,50,51 with a bridged 2ʹ-O,4ʹ-C-methylene linkage structure (Fig. 4a) for stable parallel triplex formation for gene suppression38. The bridged structure of the LNA restricts the sugar puckering to the C3ʹ-endo conformation, which induces a preorganized conformation of TFO similar to that of a triplex, resulting in increased binding stability. We incorporated LNA into the OPTO sequence and evaluated the Tm values of the LNA-incorporated OPTO (L-OPTO1–4 = L1–4). The consecutive incorporation of LNA into a TFO can decrease the thermodynamic stability of the resulting triplex48. Thus, we employed an LNA mixmer as the TFO. Additionally, we used 5mC, which has more basic N3 than cytosine, to enhance the pH-dependent thermodynamic stability of the parallel TFO52 (Fig. 4b). After incorporating LNAs and 5mCs into the TFO (L-TFO), the Tm value of the triplex containing L-TFO became 36 °C at pH 7.0, whereas the unmodified TFO did not form a triplex at pH 7.0 (Fig. 4c). Next, we investigated the effect of P at the mismatch site of L-TFO. Introducing one P at the mismatch site positioned between LNA (L-OPTO1) significantly enhanced the stability of the corresponding triplex (Tm = 45 °C). Further, introducing one P at another mismatch site between natural nucleotides (L-OPTO2) increased the stability of the corresponding triplex (Tm = 38 °C). However, the stabilizing effect of P was less pronounced compared with that in L-OPTO1. The corresponding triplex was further stabilized when P was introduced into both mismatch sites (L-OPTO3) (Tm = 50 °C). Additionally, we introduced P at both the 3ʹ and 5ʹ ends of the sequence (L-OPTO4) and measured the Tm. The normalized UV-melting curves indicated a single transition (Fig. 4c, L4), suggesting the simultaneous dissociation of L-OPTO4 and the target duplex. To confirm this, we conducted the non-denaturing polyacrylamide gel electrophoresis (Native PAGE) of triplexes (duplex/L-TFO) and (duplex/(L-OPTO1–4 = L1–4)) (Fig. 4d). The DNA samples were stained with SYBR® Gold stain (Thermo Fisher Scientific, USA) and detected as bands. The band position shifted upward proportionally to the stability of the triplex. Specifically, the triplex band of L-OPTO4 remained detectable at 37 °C, whereas that of L-OPTO3 was smeared, indicating the triplex dissociation under experimental conditions. The greater stability of the L-OPTO4 triplex compared with that of L-OPTO3 was confirmed, and the estimated Tm value of the L-OPTO4 triplex exceeded 60 °C (Supplementary Fig. S4). We also measured the annealing profile of L4 to check the hysteresis, as it is known that the folding/unfolding of a triple-helix might be not thermodynamically reversible and melting curve of the triplex are often largely shifted toward higher temperatures as compared to the annealing curve55. The superimposed data of the obtained annealing curve and melting curve of the triplex with L4 suggested that it was not completely thermodynamically reversible. However, the obtained annealing curve was still suggesting a very stable triplex formation (Supplementary Fig. S5).
a Structures of locked nucleic acid (LNA) and the conformational differences of the ribose moiety. b Sequences of the PPT duplex (606-Py/606-Pu), LNA-incorporated TFO (L-TFO), and LNA-incorporated OPTO (L-OPTO1–4). c Normalized UV-melting curves of triplexes (duplex/L-TFO) and (duplex/(L-OPTO1–4 = L1–4)) at pH 7.0 and the corresponding Tm values of the triplexes. The final concentration of duplex and TFO was 3 μM each. The Tm values are displayed as the mean ± s.d. for n = 3 replicates. d Non-denaturing polyacrylamide gel electrophoresis (Native PAGE) of triplexes (duplex/L-TFO) and (duplex/(L-OPTO1–4 = L1–4)) at pH 7.0 and 31 °C or 37 °C. The final concentration of duplex and TFO was 1 μM each. The bands were detected using SYBR® Gold stain (Thermo Fisher Scientific, USA).
Next, we investigated the impact of the positional difference of the mismatch site in the target duplex on the stability of the triplex structure to demonstrate the versatility of this approach (Fig. 5). We assessed the triplex formation using four PPT sequences, each with varying numbers of bases between two mismatch sites (Fig. 5a). The triplex formed with L-OPTO5 was significantly more stable than the corresponding LNA-incorporated TFO (L-TFO2) (Fig. 5b). For L-TFO3–5, a weak melting transition of the triplex was observed, making it challenging to accurately determine the Tm values. In contrast, the UV-melting curves of each corresponding L-OPTO6–8 displayed almost a single transition-like sigmoid curve, indicating stable triplex formation (Supplementary Fig. S4). This was further confirmed by Native PAGE analysis (Fig. 5c). We also explored the effect of the position of the LNA (Supplementary Figs. S6–7: L-OPTO9-14), revealing that the overall conformation was crucial in achieving synergistic effects (it is not necessary to introduce P between the LNA). Notably, L-OPTO13, which contained four P in its sequence, also formed a significantly stable triplex with the target duplex (Supplementary Fig. S6).
a Sequences of four PPT duplexes (Py-1/Pu-1, Py-2/Pu-2, Py-3/Pu-3, and Py-4/Pu-4), LNA-incorporated TFO (L-TFO2–5), and LNA-incorporated OPTO (L-OPTO5–8). b Normalized UV-melting curves of each triplex at pH 7.0 and their respective Tm values. The Tm values of L6-8 were determined by differential method from the region between the dotted line. The final concentration of duplex and TFO was 3 μM each. The Tm values are displayed as the mean ± s.d. for n = 3 replicates. c Native PAGE of the triplexes containing L-OPTO6–8 (L6–8) at pH 7.0 and 37 °C. The final concentration of duplex and TFO was 1 μM each. The bands were visualized using SYBR® Gold stain.
We assessed the specificity of this method by substituting mismatched pyrimidine base C with purine bases A or G. The thermodynamic stabilities of the corresponding triplexes were nearly identical (Supplementary Fig. S8), indicating that this stabilization effect did not rely on specific interactions between the bases and psoralen. Next, we exchanged the bases in the target duplex at adjacent or distal positions of the mismatch site and evaluated the thermodynamic stability of the corresponding triplex (Supplementary Fig. S9). Resultantly, the Tm values of all of the corresponding triplexes drastically decreased, indicating that the replacement of the mismatched pyrimidine base with P did not compromise the specificity of TFO.
In the other triplex stabilization method using DNA intercalator25,26, the intercalator was introduced to or replaced with nucleobases, which are capable of forming Hoogsteen hydrogen bonds (not mismatched bases). Thus, these methods potentially sacrifice the target specificity, and the target sequences contain one mismatched site at most in these experiments. Here, we showed several examples of stable parallel triplex formation with target duplex containing two mismatched sites; and the replacement of the mismatched bases shown in our method might possess an advantage in specificity as compared to others. It is noteworthy that our DNA sample solutions for the UV-melting experiment did not contain magnesium ions despite the fact that magnesium ions are often mandatory for stable triplex formation55. We also showed the importance of overall conformation of TFO to obtain a greater stabilization effect of DNA intercalator. It is reasonable to explain that conformational restriction by LNA promoted the intercalation of psoralen into DNA, resulting in the synergistic stabilization effect of L-OPTO method.
Photo-cross-linking properties of the locked nucleic acid-incorporated 1’(one)-psoralen-conjugated triplex-forming oligonucleotide
Cross-linking formation is an important factor for obtaining significant biological outcomes using TFO. We successfully demonstrated that introducing the proposed nucleotide analog (P) into TFO was effective in the stable triplex formation, which is a prerequisite factor for the photo-cross-linking of psoralen with the target duplex. Therefore, finally, we investigated the cross-linking profiles of OPTO using denaturing PAGE. As shown in Figs. 4–5, our design allows us to select sequences with multiple pyrimidine bases as the target of TFO. Therefore, we examined whether or not we could increase the photo-adduct product in proportion to the number of psoralens that was introduced into the TFO sequence (Fig. 6). Each triplex sample was irradiated using blue LED light (365 nm), and the samples were collected at each irradiation time (0, 1, 5, 10, 15, 20, 25, and 30 s). The 3’ end of each L-OPTO was labeled with tetramethylrhodamine (TAMRA). Resultantly, two clusters of the bands of the crosslinked products were observed. In the case of L-OPTO1 and L-OPTO2, we can simply define the top band clusters as monoadduct (OPTO crosslinked to either 606-Py or 606-Pu) and second band clusters as diadduct (OPTO crosslinked to both 606-Py and 606-Pu) by the mobility of the band because they have only one target site for photo-cross-linking. On the other hand, there is a possibility that crosslink formation at more than two sites restrains the extension of the DNA complex under denaturing conditions, and the band mobility will not be the same as the migration of DNA through a polyacrylamide gel depends on the end-to-end distance of the DNA. Therefore, we conducted an extensive photo-cross-linking study to analyze the composition of the photo-crosslinked product using either TAMRA-labeled 606-Py or 606-Pu; indicating that the second band clusters of photo-crosslinked product of L-OPTO3 and L-OPTO4 contained three strand complex (Supplementary Fig. S10). As we expected, the total crosslinked product formation rate increased in proportion to the number of psoralens in the L-OPTO sequence. 18% of L-OPTO1, 31% of L-OPTO3, and 47% of L-OPTO4 were consumed to form crosslinked products after 1 s of photo-irradiation. This might be attributed to both the number of psoralens in the sequence and the thermodynamic stability of the corresponding triplex. We also tested the cross-linking efficiency with other base combinations, it was observed that the efficient diadduct formation at single site required a 5ʹ-TA-3ʹ sequence in both target DNA strands (Supplementary Figs. S11–12). Generally, thymine base is more reactive than cytosine. This was experimentally examined in our previous study56,57,58. Presumably, the 5-methyl group of thymine is important for the stabilization of the radical intermediate of the [2 + 2] photo-cycloaddition reaction, and/or the hydrophobic interaction between the 5-methyl group of thymine and psoralen may contribute to the relatively high cross-linking efficiency. The photo-induced electron transfer process from the adjacent guanine base also decreases the propensity of the photo-addition of psoralen to DNA59. Generally, the target base for photo-cross-linking of psoralen-conjugated TFO is always the 5ʹ-TA-3ʹ sequence in the target DNA duplex as the cross-linking with both strand is crucial to maintain the triplex motif and to induce significant biological outcomes29,30,31,32,33,34,35,36,37,38. However, the 5ʹ-TA-3ʹ sequence doesn’t always appear like in the 5′-LTR of the HTLV-1 provirus genome. In this point, our multiple cross-linking strategy can be useful to form robust triplex motifs with those sequences without 5ʹ-TA-3ʹ. In any event, this crosslink formation is expected to enhance the biological activities of TFO upon photo-irradiation, and the crosslink forming ability distinguishes OPTO from other triplex stabilizing methods. Thus, ongoing efforts will focus on photodynamic gene regulation using the L-OPTO.
a Photo-cross-linking of psoralen with the pyrimidine base and the sequence of the PPT duplexes. The correlation between the cross-linking efficiency and the number of psoralen molecules that were introduced into the TFO sequence (L-OPTO2-4) was examined. b The photo-irradiation (365 nm) was conducted at pH 7.0 and 37 °C. The final concentration of duplex and TFO was 1 μM each. The samples were collected at each irradiation time (0, 1, 5, 10, 15, 20, 25, and 30 s), and the product formation was analyzed by denaturing P. The 3’ end of the L-OPTOs was labeled with tetramethylrhodamine (TAMRA). The graphs show the quantification results of the photo-cross-linking efficiency of each sequence. The data are displayed as the mean ± s.d. for n = 3 replicates.
Conclusion
In summary, we developed a new approach enabling stable parallel triplex formation with pyrimidine-base-interrupting sequences under physiological conditions, leveraging the synergistic effects of P and LNA and 5mC. OPTO formed stable triplexes with the 5′-LTR partial sequence of HTLV-1 and other target sequences, demonstrating the versatility of the OPTO method and its potential for expanding the range of target sequences for parallel TFO applications. Furthermore, we conducted detailed studies on the photo-cross-linking formation of OPTO and demonstrated that the internal pyrimidine base can be a target for the photo-cross-linking of psoralen. Ongoing efforts will focus on further optimizing this method and exploring photodynamic gene regulation using L-OPTO, with results expected in upcoming reports.
Methods
Chemical synthesis
Detailed protocols for the synthesis of all compounds and oligonucleotides can be found in the Supporting Information of this article.
UV-melting profiles of the triplexes
UV-melting profiles of the duplexes and triplexes were obtained using a UV-spectrophotometer equipped with a programmed thermal controller at an increased rate of 0.5 °C/min. All measurements were performed with 10 mM sodium phosphate (pH 5.3 or 7.0) containing 0.1 mM EDTA and 200 mM NaCl. Before measurements, all the sample solutions containing the ds-DNAs and TFOs (final concentration of the sample: dsDNA and TFO were 3.0 μM each, 10 mM sodium phosphate (pH 7.0), 200 mM NaCl, 0.1 mM EDTA) were denatured by heating to 95 °C and annealed by cooling to 4 °C at 0.5 °C/min.
Triplex formation analysis using non-denaturing polyacrylamide gel electrophoresis (Native PAGE)
Sample solutions containing the ds-DNAs and TFOs (1.0 μM each, 10 mM sodium phosphate (pH 7.0), 200 mM NaCl, 0.1 mM EDTA) were denatured by heating to 95 °C and cooling to 4 °C at 0.5 °C/min. The annealed sample was diluted with 40 wt% sucrose aq (sample: 40 wt% sucrose aq = 1:4 (v/v)). The samples were analyzed with 20% native polyacrylamide gel (PAGE) containing 5 mM Mg2+ in TBM (20–23 °C, 37 °C, 120 V, 90 min). The DNA bands were stained by SYBR® Gold and the gels were transferred to imaging plates. The gel images were analyzed and quantitated using ChemDoc Touch MP (BioRad, CA, U.S.A.).
Evaluation of photo-cross-linking efficiency of L-OPTOs by denaturing polyacrylamide gel electrophoresis (PAGE)
Sample solutions containing the ds-DNAs and TAMRA-labeled L-OPTOs (1.0 μM each, 10 mM sodium phosphate (pH 7.0), 200 mM NaCl, 0.1 mM EDTA) were denatured by heating to 95 °C and cooling to 4 °C at 0.5 °C/min. The annealed solution was applied to a 368-well plate and irradiated by a UV spotlight (ZUV-C30H (365 nm); Omron Corp., Kyoto, Japan) at 37 °C. The UV irradiated samples were diluted with formamide (sample: formamide = 1:4 (v/v)). The samples were analyzed by 15% denaturing polyacrylamide gel (PAGE) containing 7 M urea and formamide (200 V, 45 min). The gels were transferred to imaging plates, and the resulting gel images were analyzed and quantitated using ChemDoc Touch MP (BioRad, CA, U.S.A.).
Data availability
The authors declare that all data supporting the findings of this study are available within the article and the Supplementary Information.
References
Li, C. et al. Triplex-forming oligonucleotides as an anti-gene technique for cancer therapy. Front. Pharmacol. 13, 1007723 (2022).
Hewett, P. W. et al. Selective inhibition of the human tie-1 promoter with triplex-forming oligonucleotides targeted to Ets binding sites. Mol. Med. 12, 8–16 (2006).
Karympalis, V., Kalopita, K., Zarros, A. & Carageorgiou, H. Regulation of gene expression via triple helical formations. Biochemistry 69, 855–860 (2004).
Young, S. L., Krawczyk, S. H., Matteucci, M. D. & Toole, J. J. Triple helix formation inhibits transcription elongation in vitro. Proc. Natl. Acad. Sci. USA 88, 10023–10026 (1991).
Moser, H. E. & Dervan, P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science 238, 645–650 (1987).
Faruqi, A. F., Seidman, M. M., Segal, D. J., Carroll, D. & Glazer, P. M. Recombination induced by triple-helix-targeted DNA damage in mammalian cells. Mol. Cell. Biol. 16, 6820–6828 (1996).
Kaushik Tiwari, M. & Rogers, F. A. XPD-dependent activation of apoptosis in response to triplex-induced DNA damage. Nucleic Acids Res. 41, 8979–8994 (2013).
Kaushik Tiwari, M. et al. Direct targeting of amplified gene loci for proapoptotic anticancer therapy. Nat. Biotechnol. 40, 325–334 (2022).
Kaushik Tiwari, M., Adaku, N., Peart, N. & Rogers, F. A. Triplex structures induce DNA double strand breaks via replication fork collapse in NER deficient cells. Nucleic Acids Res. 44, 7742–7754 (2016).
Nagatsugi, F., Sasaki, S., Miller, P. S. & Seidman, M. M. Site-specific mutagenesis by triple helix-forming oligonucleotides containing a reactive nucleoside analog. Nucleic Acids Res. 31, e31 (2003).
Mikame, Y. & Yamayoshi, A. Recent advancements in development and therapeutic applications of genome-targeting triplex-forming oligonucleotides and peptide nucleic acids. Pharmaceutics 15, 2515 (2023).
Torigoe, H., Hari, Y., Sekiguchi, M., Obika, S. & Imanishi, T. 2’-O,4’-C-methylene bridged nucleic acid modification promotes pyrimidine motif triplex DNA formation at physiological pH: thermodynamic and kinetic studies. J. Biol. Chem. 276, 2354–2360 (2001).
Hartono, Y. D. et al. Role of pseudoisocytosine tautomerization in triplex-forming oligonucleotides: In silico and in vitro studies. ACS Omega 2, 2165–2177 (2017).
Rusling, D. A. Triplex-forming properties and enzymatic incorporation of a base-modified nucleotide capable of duplex DNA recognition at neutral pH. Nucleic Acids Res. 49, 7256–7266 (2021).
Verma, S. & Miller, P. S. Interactions of cytosine derivatives with T·A interruptions in pyrimidine·purine·pyrimidine DNA triplex. Bioconjugate Chem. 7, 600–605 (1996).
Guianvarc’h, D., Benhida, R., Fourrey, J. L., Maurisse, R. & Sun, J. S. Incorporation of a novel nucleobase allows stable oligonucleotide-directed triple helix formation at the target sequence containing a purine·pyrimidine interruption. Chem. Commun. 18, 1814–1815 (2001).
Ohkubo, A., Ohnishi, T., Nishizawa, S., Nishimura, Y. & Hisamatsu, S. The ability of a triplex-forming oligonucleotide to recognize T-A and C-G base pairs in a DNA duplex is enhanced by incorporating N-acetyl-2,7-diaminoquinoline. Bioorg. Med. Chem. 28, 115350 (2020).
Gerrard, S. R., Edrees, M. M., Bouamaied, I., Fox, K. R. & Brown, T. CG base pair recognition within DNA triple helices by modified N-methylpyrrolo-dC nucleosides. Org. Biomol. Chem. 8, 5087–5096 (2010).
Hari, Y., Akabane, M. & Obika, S. 2’-4’-BNA bearing a chiral guanidinopyrrolidine-containing nucleobase with potent ability to recognize the C:G base pair in a parallel-motif DNA triplex. Chem. Commun. 49, 7421–7423 (2013).
Obika, S. et al. 2’-O,4’-C-methylene bridge nucleic acid (2’,4’-BNA): Synthesis and triplex-forming properties. Bioorg. Med. Chem. 9, 1001–1011 (2001).
Sasaki, S. et al. Selective formation of stable triplexes including a TA or a CG interrupting site with new bicyclic nucleoside analogue (WNA). J. Am. Chem. Soc. 126, 516–528 (2004).
Inde, T. et al. Synthesis of and triplex formation in oligonucleotides containing 2’-deoxy-6-thioxanthosine. Bioorg. Med. Chem. 26, 3785–3790 (2018).
Wang, E., Koshlap, K. M., Gillespie, P., Dervan, P. B. & Feigon, J. Solution structure of a pyrimidine·purine·pyrimidine triplex containing the sequence-specific intercalating non-natural base D3. J. Mol. Biol. 257, 1052–1069 (1996).
Carbone, G. M. et al. DNA binding and antigene activity of a daunomycin-conjugated triplex-forming oligonucleotide targeting the P2 promoter of the human c-myc gene. Nucleic Acids Res. 32, 2396–2410 (2004).
Paramasivam, M. et al. Purine twisted-intercalating nucleic acids: a new class of anti-gene molecules resistant to potassium-induced aggregation. Nucleic Acids Res. 36, 3494–3507 (2008).
Walsh, S., El-Sagheer, A. H. & Brown, T. Fluorogenic thiazole orange TOTFO probes stabilize parallel DNA triplexes at pH 7 and above. Chem. Sci. 9, 7681–7687 (2018).
Kumar, V., Kesavan, V. & Gothelf, K. V. Highly stable triple helix formation by homopyrimidine (L)-acyclic threoninol nucleic acids with single stranded DNA and RNA. Org. Biomol. Chem. 13, 2366–2374 (2015).
Buhimschi, D. A. et al. Psoralen derivatives with enhanced potency. Photochem. Photobiol. 96, 1014–1031 (2020).
Duval-Valentin, G., Thuong, N. T. & Hélène, C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc. Natl. Acad. Sci. USA 89, 504–508 (1992).
Grigoriev, M. et al. Inhibition of gene expression by triple helix-directed DNA cross-linking at specific sites. Proc. Natl. Acad. Sci. USA. 90, 3501–3505 (1993).
Havre, P. A., Gunther, E., Gasparro, F. & Glazer, P. M. Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proc. Natl. Acad. Sci. USA 90, 7879–7883 (1993).
Raha, M., Lacroix, L. & Glazer, P. M. Mutagenesis mediated by triple helix-forming oligonucleotides conjugated to psoralen: effects of linker arm length and sequence context. Photochem. Photobiol. 67, 289–294 (1998).
Faria, M. et al. Targeted inhibition of transcription elongation in cells mediated by triplex-forming oligonucleotides. Proc. Natl. Acad. Sci. USA 97, 3862–3867 (2000).
Diviacco, S. et al. Site-directed inhibition of DNA replication by triple helix formation. FASEB J. 15, 2660–2668 (2001).
Majumdar, A. et al. Targeted gene knock-in and sequence modulation mediated by a psoralen-linked triplex-forming oligonucleotide. J. Biol. Chem. 283, 11244–11252 (2008).
Liu, J., Majumdar, A., Liu, J., Thompson, L. H. & Seidman, M. M. Sequence conversion by single strand oligonucleotide donors via non-homologous end joining in mammalian cells. J. Biol. Chem. 285, 23198–23207 (2010).
Semenyuk, A. et al. Targeting of an interrupted polypurine:polypyrimidine sequence in mammalian cells by a triplex-forming oligonucleotide containing a novel base analogue. Biochemistry 49, 7867–7878 (2010).
Mikame, Y. et al. Development and crosslinking properties of psoralen-conjugated triplex-forming oligonucleotides as antigene tools targeting genome DNA. ChemMedChem 18, e202300348 (2023).
Li, H. et al. Triplex staples: DNA double-strand cross-linking at internal and terminal sites using psoralen-containing triplex-forming oligonucleotide. Bioconjugate Chem. 17, 1561–1567 (2006).
Nagai, M. & Osame, M. Human T-cell lymphotropic virus type I and neurological diseases. J. Neurovirol. 9, 228–235 (2003).
Watanabe, T. HTLV-1-associated diseases. Int. J. Hematol. 66, 257–278 (1997).
Yasunaga, J. & Matsuoka, M. Molecular mechanisms of HTLV-1 infection and pathogenesis. Int. J. Hematol. 94, 435–442 (2011).
Faris, M. Potential for molecular targeted therapy for adult T-cell leukemia/lymphoma. Int. Rev. Immunol. 27, 71–78 (2008).
Taylor, G. P. & Matsuoka, M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 24, 6047–6057 (2005).
Tanaka, A. et al. A novel therapeutic molecule against HTLV-1 infection taregeting provirus. Leukemia 27, 1621–1627 (2013).
Wang, T. T. et al. Current state of therapeutics for HTLV-1. Viruses 16, 1616 (2024).
Inoue, J. et al. Nucleotide sequence of the protease-coding region in an infectious DNA of simian retrovirus (STLV) of the HTLV-I family. Virology 150, 187–195 (1986).
Obika, S. et al. 2’-O,4’-C-methylene bridged nucleic acid (2’,4’-BNA): synthesis and triplex-forming properties. Bioorg. Med. Chem. 9, 1001–1011 (2001).
Brunet, E. et al. Exploring cellular activity of locked nucleic acid-modified triplex-forming oligonucleotides and defining its molecular basis. J. Biol. Chem. 280, 2007–20086 (2005).
Højland, T. et al. LNA (locked nucleic acid) and analogs as triplex-forming oligonucleotides. Org. Biomol. Chem. 5, 2375–2379 (2007).
Danielsen, M. B. et al. Polyamine-functionalized 2ʹ-amino-LNA in oligonucleotides: facile synthesis of new monomers and high-affinity binding towards ssDNA and dsDNA. Chem. Eur. J. 27, 1416–1422 (2021).
Lee, J. S., Woodsworth, M. L., Latimer, L. J. & Morgan, A. R. Poly(pyrimidine)·poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 12, 6603–6614 (1984).
Jazouli, M. et al. A short and efficient synthesis of 2ʹ-deoxybenzo and pyridoimidazole C-nucleosides. Tetrahedron Lett. 44, 5807–5810 (2003).
Martín-Nieves, V. et al. Oligonucleotides containing 1-aminomethyl or 1-mercaptomethyl-2-deoxy-D-ribofuranoses: synthesis, purification, characterization, and conjugation with fluorophores and lipids. Bioconjugate Chem. 32, 350–366 (2021).
Francois, J. S., Lacoste, J., Lacroix, L. & Mergny, J. L. Design of antisense and triplex forming oligonucleotides. Methods Enzymol. 313, 74–95 (2000).
Yamayoshi, A., Matsuyama, Y., Kushida, M., Kobori, A. & Murakami, A. Novel photodynamic effect of a psoralen-conjugated oligonucleotide for the discrimination of the methylation of cytosine in DNA. Photochem. Photobiol. 90, 716–722 (2014).
Kojima, A. et al. Selective photo-crosslinking detection of methylated cytosine in DNA duplex aided by a cationic comb-type copolymer. ACS Biomater. Sci. Eng. 8, 1799–1805 (2022).
Nakao, J. et al. Unique crosslinking properties of psoralen-conjugated oligonucleotides developed by novel psoralen N-hydroxysuccinimide esters. ChemBioChem 24, e202200789 (2023).
Bertling, J. et al. Synthesis and photophysics of water-soluble psoralens with red-shifted absorption. Photochem. Photobiol. 97, 1534–1547 (2021).
Acknowledgements
This study was financially supported by the Grant-in-Aid for Transformative Research Areas (A) “Material Symbiosis” (Grant Number:20H05874 awarded to A.Y.) from MEXT, Japan. This study was also supported by JSPS KAKENHI (Grant Numbers 22H00593 and 22K14839 to A.Y. and Y.M., respectively), Japan, AMED (Grant Number JP24ak0101227h0001 to A.Y) and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” (Grant Numbers 20224030 and 20228004 to A.Y. and Y.M., respectively).
Author information
Authors and Affiliations
Contributions
Y.M. and A.Y. conceptualized and supervised the project with the assistance of C.D. and T.W. Y.M. synthesized all compounds. The functional evaluation experiments of OPTO were performed by Y.M. and H.T. The manuscript was written with the contribution of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Vyacheslav V. Filichev and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mikame, Y., Toyama, H., Dohno, C. et al. Development and functional evaluation of a psoralen-conjugated nucleoside mimic for triplex-forming oligonucleotides. Commun Chem 8, 18 (2025). https://doi.org/10.1038/s42004-025-01416-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-025-01416-2