Abstract
Background
Adrenoleukodystrophy (ALD) is associated with disruption in very long chain fatty acid (VLCFA) processing due to a genetic mutation in the X-linked ABCD1 gene. The buildup of VLCFAs predisposes males to adrenal insufficiency (AI) and cerebral demyelination (cerebral ALD). If diagnosed early, these conditions can be treated effectively by hormone replacement and hematopoietic stem cell transplant, respectively. ALD is now included on the newborn screen panel in the majority of the United States. Thus far, there are no predictive tools to identify the development of early cerebral disease, necessitating frequent imaging surveillance with biannual MRIs starting at age 2 years through 12 years, then per annum for the remainder of the patient’s life. A bloodborne biomarker has been desirable to efficiently predict the onset of cerebral disease and reduce the healthcare burden on the family as well as healthcare expense.
Methods
We prospectively measured plasma neurofilament light (NfL) chain levels in five boys with ALD who ultimately developed cerebral ALD.
Results
We describe that baseline plasma NfL levels can be established for boys with ALD and that an increase of 50% above baseline occurs at the time that cerebral disease develops.
Conclusions
As far as we are aware, this is the first report highlighting that a bloodborne biomarker, plasma NfL, could potentially be used as a concomitant indicator of cerebral disease initiation, thus simplifying surveillance for cerebral ALD.
Plain language summary
Adrenoleukodystrophy (ALD) is an inherited disorder that affects how the body processes very long chain fatty acids (VLCFAs). In boys, this can lead to problems with the adrenal glands and damage to the brain. ALD is commonly found through newborn screening. Currently doctors must monitor boys with ALD using frequent blood tests and brain scans from age 2 and throughout life to screen for the development of disease in the brain. A blood test that could screen for disease development would be helpful for families and doctors as it would be easier to carry out than imaging. We find that the amount of a component in the blood called neurofilament light chain could be an indicator of the development of disease in the brain. This could provide an easier method to screen for the development of disease in the brain.
Similar content being viewed by others
Introduction
ALD is an X-linked disorder caused by mutations in the ABCD1 gene that encodes an ABC class very long chain fatty acid (VLFCA) transporter known as ALD protein (ALDP). ALDP transports VLCFA from the cytoplasm to the peroxisome for breakdown. Lack of function of this transport activity leads to VLCFA buildup at the cellular and tissue levels, eventually resulting in cell death and organ dysfunction1. The most sensitive organ is the adrenal gland which results in adrenal insufficiency (AI) in boys often between ages four and seven with a rate of 50% or greater by age 18 years2. Approximately 40% of boys develop a devastating inflammatory cerebral demyelination process known as cerebral ALD. Once initiated, this process rarely self-arrests, and the best known current medical treatment is either a hematopoietic stem cell transplant (HSCT) from a sufficiently matched donor or ex-vivo lentiviral gene therapy3,4. Given the substantial incidence of cerebral ALD, international guidelines have been established for longitudinal monitoring of boys with an ALD diagnosis5. Cerebral disease surveillance consists of initiation of MRIs at about age 2 years, which are then performed every six months until age 12, whereafter they occur annually for the remainder of the patient’s life. In young children and toddlers, the MRIs are often performed with sedation and may include the use of gadolinium contrast enhancement to identify inflammatory lesions. While MRI screening is generally safe, there are still some risks associated with pediatric sedation, and there is ongoing debate regarding long term effects of gadolinium contrast infusion6,7,8,9,10,11.
The search for a biomarker to predict the onset of cerebral ALD has been ongoing for the better part of two decades. Numerous biomarkers have been reported including metalloproteinase levels12, profilin13, chitotriosidase14, and others which have been associated with cerebral ALD. Recently there’s been more focus on the use of neurofilament light chain (NfL) as a biomarker given the newfound ability to detect it in the blood at subfemtomolar levels due to the development of the highly sensitive single-molecule enzyme-linked immunosorbent assay (Simoa) methodology15. NfL has been utilized in numerous neurologic disorders to monitor disease progression and response to therapy16,17,18. Recent published work has demonstrated that NfL is associated with cerebral ALD as well as response to therapy (HSCT)19. The use of NfL has not been explored prospectively as a longitudinal biomarker to detect cerebral disease.
In this report, we describe that baseline plasma NfL levels can be established for boys with ALD. We show that an increase of 50% above baseline occurs at the time that cerebral disease develops as shown by MRI. As far as we are aware, this is the first report highlighting that a bloodborne biomarker, plasma NfL, could potentially be used as a concomitant indicator of cerebral disease initiation, thus simplifying surveillance for cerebral ALD.
Methods
All patients were assessed at the University of Minnesota Leukodystrophy Center of Excellence according to standard of care guidelines at our institution. This study and the use of all patient samples were approved by the Institutional Review Board at the University of Minnesota, (protocol code 0808M4232, date of last approval: 9/30/2024, Adrenoleukodystrophy National Registry and Biorepository, NCT03789721, lasted approved 01/15/2025). Parents/guardians gave informed consent for their children’s blood samples to be collected and analyzed; children over age 8 years gave assent. Samples were collected in a standard purple top blood collection tube (containing ethylenediaminetetraacetic acid (EDTA), as an anticoagulant); plasma was isolated within 6 h of collection and stored at −150 °C in a vapor phase liquid nitrogen cryogenic storage freezer. NfL was measured by Quanterix (Billerica, MA) using Simoa methodology.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Five boys with ALD were monitored longitudinally with plasma analysis and MRIs, but none of them have developed cerebral disease. Figure 1 shows their NfL levels over time which varied between 2.1 and 8.9 pg/mL for the cohort; the coefficient of variation within an individual varied from 8.9% to 38.0% with a median of 16% (Fig. 1). A sixth boy had only a single timepoint at the time of this analysis.
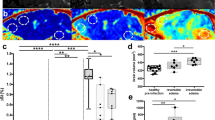
We next identified five boys with ALD who ultimately developed cerebral disease proven by demyelination lesions present on MRI, for whom there was at least one baseline plasma sample with a normal MRI prior to cerebral disease activation. Figure 2a shows Patient 1 with a pre-lesion NfL level of 3.5 pg/mL followed by levels that rose by 121% at the time of lesion development. The initial lesion showed gadolinium enhancement 1 year after its initial development. Figure 2b shows a boy with a new lesion at nearly 12 years of age whose pre-lesion NfL was 2.1 pg/mL which had increased by 196% at lesion development (which also had gadolinium enhancement). The boy in Fig. 2c developed cerebral disease at 3.9 years of age which initially was gadolinium “equivocal” in terms of enhancement but then showed unequivocal enhancement on follow-up MRI 3 months later. The rise in NfL above baseline was 58% at new lesion development and 149% when there was clear contrast enhancement detected. Figure 2d shows NfL levels in a 5-year-old boy with several pre-lesional samples which varied from 3.5 to 4.5 pg/mL. The initial lesion was determined as “equivocal” in terms of gadolinium enhancement and then later (6 months) converted to discrete contrast enhancement; the change above average baseline was 65% and 102%, respectively. Figure 2e shows a 5-year-old boy with a new gadolinium enhancing lesion and increase in NfL 399% above his average baseline level (6.03 pg/mL) that was established with five serial samples prior to any cerebral disease development.
a–e shows five boys with baseline plasma NfL levels and normal MRIs prior to development of cerebral disease. Timepoints shown were chosen to indicate the association of lesion development with the rise in NfL. Change from mean baseline NfL level is indicated in each graph. Some boys had a single baseline timepoint while others had multiple pre-lesional samples. The score indicates the overall Loes MRI score for the T2-weighted MRI sequences obtained. Red boxes indicate magnified areas. Blue arrows indicate areas of gadolinium contrast enhancement.
The above data show that lesion development is associated with a substantial spike in NfL. The identification of overt blood-brain-barrier (BBB) disruption identified by gadolinium enhancement was associated with a further elevation of NfL in two of the boys, which is of interest. To explore this latter point in more detail, we assessed two brothers with advanced cerebral ALD (Loes MRI score >14) where one brother (Fig. 3a) had an arrested state of cerebral ALD (no gadolinium enhancement was present) and the other showed active cerebral ALD indicated by the presence of gadolinium enhancement (Fig. 3b). Both boys had elevated NfL levels compared to boys without cerebral ALD (Fig. 3), but the boy with gadolinium positivity had NfL 10-fold higher than his sibling, suggesting that overt BBB breakdown enhances the level of NfL in the peripheral circulation (Fig. 3).
Two brothers were diagnosed with cerebral ALD in a similar time frame. a The left images show the brother with arrested cerebral disease (no gadolinium enhancement on lower image). b The right images show the brother with active cerebral disease indicated by gadolinium enhancement (white arrow). Plasma NfL levels at the time of MRI are indicated.
Discussion
This study shows the potential of NfL as a suggestive biomarker of cerebral ALD conversion in boys with ALD. We find a greater than 50% elevation from baseline NfL concentration to be very robust in indicating the development of a cerebral lesion (cerebral ALD) which was +/− contrast enhancement, although overt contrast enhancement was associated with a much higher elevation in NfL. This is an important finding, as active cerebral disease defined by the development of a demyelinating lesion is an indication to consider HSCT or gene therapy to arrest the disease process. In addition, given there are some concerns about sedation in very young children and potential long-term effects due to gadolinium, a heavy metal, exposure, NfL may prove to be an important surrogate biomarker of cerebral disease activation.
There are several aspects of NfL that need to be recognized. First, there is a normal decline in NfL levels as a newborn develops from birth to 10 years as shown in the large population study by Abdelhak et al. 20. Although our data does not necessarily reflect that experience, the numbers in our study are relatively small and specific to these boys with ALD. Despite any downward levels of NfL that could occur in the in the first 10 years of life, our data show that at cerebral disease development, there is an abrupt increase in NfL levels.
Another consideration is that the concentration of plasma NfL can vary. Sources of variability include laboratory sample processing and measurement. Ideally, preserving sample integrity comes from collecting the same type of sample (plasma not serum in this study) and storing it in a similar fashion (liquid nitrogen). Method of measurement should be the same for an individual patient (Simoa vs. microfluidic immunoassay, for example). Perhaps future studies could determine another CNS-originating biomarker akin to a house-keeping protein that can normalize the CNS release of NfL to account for variability in NfL plasma concentration. There are also genetic differences associated with BBB integrity; well-known examples are the APOE gene variants, specifically, APOE4 which is associated with a reduction in BBB integrity21. Interestingly, we have reported APOE4 variant carriers to be associated with the development of worse cerebral disease in ALD compared to non-APOE4 carriers22. Whether a particular APOE variant correlates to [NfL] independently is not known but could be investigated in future cohorts. Finally, small but significant increases in plasma NfL have been associated with head trauma in sports17. While young children are not yet playing competitive sports, the occasional fall and “head-bonk” is inevitable; this can be revealed through careful clinical history taking at surveillance visits.
We should also consider the indirect nature of plasma NfL measurement as a marker reflecting the dynamics of the central nervous system, and there are reports measuring NfL in cerebrospinal fluid (CSF), which is considered to more directly reflect central nervous system (CNS) dynamics. As an example, recently Kakumoto et al. measured CSF NfL levels finding elevated levels in patients with cerebral ALD compared to those with AMN23. In two of four patients that converted to active cerebral ALD while being followed longitudinally, the CSF NfL levels increased >50% which is similar in response to our data. Being that plasma is easier to sample compared with CSF it was initially important to establish a good (though not perfect) correlation between CSF and plasma levels which has been done16,19. Although CSF NfL may be closer to the source of cellular damage, the ability of NfL to cross the BBB and enter the plasma makes it a more accessible biomarker and given that most patients would unlikely want to a undergo lumbar puncture every 6 months (the interval for pediatric cerebral disease monitoring), a more desirable biomarker for patients as well.
Taking the above arguments into consideration, establishing a cut-off value in terms of a uniform pg/mL concentration may be challenging. Related to this, the absolute NfL level may vary considerably from one patient to another. We note the maximal coefficient of variability we observed in our ALD cohort without cerebral disease was 37%. It appears therefore important to establish, for any individual, an average baseline NfL concentration based on serial measurements. A subsequent rise >50% above the baseline NfL could indicate the development of a cerebral lesion as suggested by our data.
Our small cohort results are in agreement with prior work of Weinhofer et al. who showed a significant increase from baseline NfL in two adults when they converted to active cerebral ALD, though their baseline levels were established >4 years before cerebral ALD activation24. It is imperative that these pilot studies be expanded in larger patient cohorts to verify these findings. This will take cooperation between large ALD centers of expertise to collect the MRI data as well as obtaining consistent plasma samples in a similar manner. Verification of NfL association with cerebral disease development may lead to a reduction in sedated MRIs for young children, less gadolinium exposure, less time in the health facility for a family, and overall healthcare monetary savings for families.
Data availability
A de-identified dataset that underlies the results reported in this article may be made available on reasonable request to academic investigators without commercial employment for the purpose of verifying and replicating results.
References
Yska, H. A. F., Engelen, M. & Bugiani, M. The pathology of X-linked adrenoleukodystrophy: tissue specific changes as a clue to pathophysiology. Orphanet J. Rare Dis. 19, 138 (2024).
Regelmann, M. O. et al. Adrenoleukodystrophy: guidance for adrenal surveillance in males identified by newborn screen. J. Clin. Endocrinol. Metab. 103, 4324–4331 (2018).
Eichler, F. et al. Lentiviral gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 391, 1302–1312 (2024).
Miller, W. P. et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood 118, 1971–1978 (2011).
Engelen, M. et al. International recommendations for the diagnosis and management of patients with adrenoleukodystrophy: a consensus-based approach. Neurology 99, 940–951 (2022).
Davidson, A. J. et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387, 239–250 (2016).
de Graaff, J. C., Johansen, M. F., Hensgens, M. & Engelhardt, T. Best practice & research clinical anesthesiology: safety and quality in perioperative anesthesia care. Update on safety in pediatric anesthesia. Best Pr. Res. Clin. Anaesthesiol. 35, 27–39 (2021).
Fraum, T. J., Ludwig, D. R., Bashir, M. R. & Fowler, K. J. Gadolinium-based contrast agents: a comprehensive risk assessment. J. Magn. Reson. Imaging 46, 338–353 (2017).
Rozenfeld, M. N. & Podberesky, D. J. Gadolinium-based contrast agents in children. Pediatr. Radio. 48, 1188–1196 (2018).
Golec, W., Jakimow-Kostrzewa, A., Mruk, B. & Jozwiak, S. Role of gadolinium-based contrast agents in neurological disorders. Neurol. Neurochir. Pol. 54, 399–409 (2020).
Unruh, C., Van Bavel, N., Anikovskiy, M. & Prenner, E. J. Benefits and detriments of gadolinium from medical advances to health and ecological risks. Molecules 25, 5762 (2020).
Thibert, K. A. et al. Cerebrospinal fluid matrix metalloproteinases are elevated in cerebral adrenoleukodystrophy and correlate with MRI severity and neurologic dysfunction. PLoS O NE 7, e50430 (2012).
Orchard, P. J. et al. Cerebral adrenoleukodystrophy is associated with loss of tolerance to profilin. Eur. J. Immunol. 49, 947–953 (2019).
Orchard, P. J. et al. Chitotriosidase as a biomarker of cerebral adrenoleukodystrophy. J. Neuroinflamm. 8, 144 (2011).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010).
Disanto, G. et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870 (2017).
Shahim, P. et al. Neurofilament light as a biomarker in traumatic brain injury. Neurology 95, e610–e622 (2020).
Nitz, E. et al. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann. Clin. Transl. Neurol. 8, 2013–2024 (2021).
Wang, H. et al. Evaluation of neurofilament light chain as a biomarker of neurodegeneration in X-linked childhood cerebral adrenoleukodystrophy. Cells 11, 913 (2022).
Abdelhak, A. et al. Serum neurofilament light chain reference database for individual application in paediatric care: a retrospective modelling and validation study. Lancet Neurol. 22, 826–833 (2023).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Orchard, P. J. et al. Association between APOE4 and biomarkers in cerebral adrenoleukodystrophy. Sci. Rep. 9, 7858 (2019).
Kakumoto, T. et al. Neurofilament light chain levels in cerebrospinal fluid as a sensitive biomarker for cerebral adrenoleukodystrophy. Ann. Clin. Transl. Neurol. 10, 1230–1238 (2023).
Weinhofer, I. et al. Neurofilament light chain as a potential biomarker for monitoring neurodegeneration in X-linked adrenoleukodystrophy. Nat. Commun. 12, 1816 (2021).
Acknowledgements
We gratefully acknowledge our funding sources for their contributions: The Hall Family, Oliver’s Army, X out ALD, and Knockout ALD.
Author information
Authors and Affiliations
Contributions
T.C.L. designed the study and wrote the manuscript. D.J.L. and D.R.N. contributed to data collection. A.O.G. and P.J.O. provided expert advice and manuscript editing. All authors contributed to the interpretation of the results and the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: T.C.L. has consulted for and served as an investigator for bluebird bio and Minoryx Therapeutics. A.O.G., P.J.O., and D.R.N. have consulted or served as an investigator for Bluebird Bio. D.J.L. has consulted for Bluebird Bio, Minoryx Therapeutics, and Swanbio.
Peer review
Peer review information
Communications Medicine thanks Takashi Matsukawa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lund, T.C., Gupta, A.O., Loes, D.J. et al. Neurofilament light chain as a biomarker to indicate early activation of cerebral disease in boys with adrenoleukodystrophy. Commun Med 5, 196 (2025). https://doi.org/10.1038/s43856-025-00915-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-00915-6