Abstract
Alkaline water electrolysers are ideal for gigawatt-scale hydrogen production due to the usage of non-precious metal and low-cost raw materials. However, their performances are modest with the separated electrode and diaphragm structure which can date back to more than 100 years ago. Here we report a catalyst-coated diaphragm assembly to improve the performance of alkaline water electrolysers. The transport resistance of OH- ions is reduced and the electrochemical surface area of catalysts is enlarged by more than forty fold, representing more than 40% increase in hydrogen production rate or as much as 16% reduction in energy consumption. The electrolyser with our catalyst-coated diaphragm assembly delivers current densities as high as 1 A cm−2 at 1.8 V or 2 A cm−2 at 2 V and shows good stability after more than 1000 hours of operation. Therefore, the catalyst-coated diaphragm assembly route is promising for the development of high-performance and efficient alkaline water electrolysers.
Similar content being viewed by others
Introduction
Hydrogen is a key factor for future sustainability, acting as clean energy medium for transportation, industry and stationary power, as well as green feed-stocks for chemicals and materials1. Hydrogen production using water electrolysis is projected to reach 550 million tones by 2050, contributing to about 20% of CO2 abatement2. To reach this scale in its production, alkaline water electrolysers (AWEs) will play a critical role because the component materials used in AWEs are abundant and less costly for hundreds of gigawatts implementations. To the contrary, the proton exchange membrane electrolyser (PEME) with a similar technical readiness uses scarce and costly platinum group metal electrocatalysts3,4. However, the AWE has its own drawbacks to be solved, i.e., the efficiency loss due to the high electrode overpotential and ohmic resistance, primarily caused by the large gap between cathode and anode. This gap leads to low utilization of electrocatalyst and long OH- transport distance5. Therefore, electrolyser structures with zero-gap of electrode and diaphragm were developed6 and thin diaphragm of ZrO2 coated polyphenylene sulfide mesh, e.g., Zirfon with a thickness of 220 μm, was used to improve the performance of AWEs7. Thus far, the overvoltage of AWEs is still about 200 mV higher than that of PEMEs, and AWE’s current density is less than 25% of that of a PEME7. These translate into about 10% lower in efficiency and more than 4 times larger in the electrolyser’s volume.
The performance difference between AWE and PEME stems from the different electrolytes used. Unlike PEME, the AWE cathode is immersed in liquid alkali solutions and the hydrogen evolution reaction (HER) encounters additional bubble overpotential, due to the reduction of fresh electrocatalytic sites caused by the bubble coverage. The HER bubble overpotential is estimated to be about 45 mV, with bubble coverage at about 90% and Tafel slop of 45 mV dec-1 for cathode electrocatalysts8,9. Moreover, the mobility of OH- is nearly half of that of H+, resulting in higher ionic resistance in an AWE than that in a PEME10,11. The proton conductivity of the state-of-the-art Nafion membrane for PEMEs is measured at higher than 21.5 S m-1, while the ion conductivity of Zirfon diaphragm for AWEs is about 15 S m-1, adding to about 40 mV of ohmic overpotential for an AEW at a current density of 1 A cm−2 compared with a PEME with identical membrane thickness12,13. Considering the more favorable oxygen evolution reaction (OER) in AWEs than in PEMEs14, the performance of AWEs should be close to that of PEMEs, with no more than 100 mV higher in cell voltage. Nevertheless, the state-of-the-art AWEs usually require a single cell voltage of almost 2 V at a current density below 0.5 A cm−2 15,16,17, quite a drawback in performance compared with that of a PEME, i.e., cell voltage of about 2 V at a current density of 2 A cm−2 18,19.
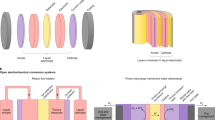
We postulate that the primary reason in the suppressed efficiency of an industrial AWE is in the hundred years old cell structure itself with separated electrode and diaphragm (SED) as shown in Fig. 1a, b. Even though the electrode is physically pressed onto the diaphragm for the zero-gap AWE, there is still micro gap between the electroctalyst and the ion transport interface. As a result, the bubbles in the gap disturb the electric field and block the OH- transport in the electrolyte20,21, exhibiting rather high ohmic resistance and hindering the electrochemical reaction. On the contrast, the concept of membrane electrode assembly is widely used in PEMEs ever since 1950s22,23, with nanostructured catalyst coated on the membrane using proton exchange ionomer as the binder, leading to improved electrolysis performance by enlarged electrochemical reaction area and reduced H+ transport distance. The membrane electrode assembly route is also introduced to the development of anion exchange membrane electrolyser, which is considered as a new type of alkaline electrolyser24. However, the membrane electrode assembly route is seldom experimented for AWEs by coating electrocatalysts onto the porous diaphragm, and only marginally SED-comparable performance was reported by very few works25.
Here, we demonstrated a substantial performance improvement of an industrial AWE, i.e., single cell voltage of 2 V at a current density of 2 A cm−2, comparable with that of the state-of-the-art PEME, by introducing the catalyst-coated diaphragm (CCD) assembly concept to fabricate the electrolyser as shown in Fig. 1c, d. We achieved a 40-fold electrocatalyst utilization and a 40% reduction in ohmic resistance compared with the conventional SED AWEs, due to the realization of direct electrocatalyst-diaphragm contact at micro scale level. Most of the raw materials used for this CCD route AWE are the same as those used in the state-of-the-art AWEs, making it possible in its quick implementation to industrial applications. We believe that the transition from SED to CCD would benefit the development of high-performance and efficient AWEs, contributing to large scale production of green hydrogen for future sustainability.
Results
Structural differences between the CCD and SED
The microstructure of the CCD was characterized for the catalyst layer and the cross section. The scanning electron microscope (SEM) image in Fig. 2a shows that the CCD catalyst layer exhibits random agglomerates of Raney Ni particles with featured size of between sub-10 and 30 μm. The high resolution transmission electron microscope (HR-TEM) image shown in Fig. 2b indicates that the Nafion binder is spread on the surface of the Raney Ni particle, and the Nafion layer thickness is measured at about 5 nm, which is similar to the case of typical fuel cell catalyst layer using carbon supported Pt (Pt/C) as the catalyst26. Ultra-depth optical microscope image in Fig. 2c reveals the rough nature of the CCD surface, with catalyst layer thickness ranging from 5 to 10 μm. Vacancies are also created among the Nafion-bindered agglomerates of Raney Ni particles, which is attributed to the uneven distribution of the catalyst particles during the blade coating process. Cross section image (Fig. 2d) and elemental mapping (Fig. 2e) of the CCD show that catalyst layers are directly bound to each side of the diaphragm, and stacking pores are formed among the Raney Ni particles, which makes the transport channel for the electrolyte as well as the gases produced. The statistical porosity of the CCD is further measured using mercury intrusion method, with results shown in Fig. 2f. For pristine Zirfon 220 diaphragm, dominant pores are located between the diameter of 50 nm and 500 nm, with minor pores located around 10 μm. The pore volume peaks are detected at pore diameters of 60 nm, 150 nm and 10 μm, showing mesoporous nature of the Zirfon 220 diaphragm. As for the fresh Raney Ni catalsyt, most of the pores are located around the diameter of 2 μm. When Nafion-bindered Raney Ni particles are coated onto both sides of the Zirfon 220 diaphragm to form the CCD, the pores with diameters of between 100 nm and 400 nm are reduced, and the pore volume peak located at 10 μm also declined. The particle size of Raney Ni catalyst is larger than 2 μm, and the catalyst loading is only 2 mg cm−2, much lower than the areal density of Zirfon 220 diaphragm (~22 mg cm−2). As a result, the slightly increased porosity of the CCD at 0.64 over Zirfon 220 diaphragm’s 0.60 is ascribed to the formation of pores with the diameter located between 11 and 13 μm which are probably caused by the packing of Raney Ni particles and Nafion ionomer aggregates27.
a Representative scanning electron microscope image of the Raney Ni catalyst layer. b High resolution transmission electron microscope image of typical Raney Ni particle in the catalyst-coated diaphragm. c Representative ultra-depth optical microscope image of the catalyst-coated diaphragm with Raney Ni catalyst layer. d, e Typical cross-section scanning electron microscope image (d) and elemental mapping (e) of the catalyst-coated diaphragm. f Pore diameter distributions of Zirfon 220 diaphragm, Raney Ni catalyst and the catalyst-coated diaphragm. The pore volume is normalized to the sample mass of the catalyst-coated diaphragm. g, h Scanning electron microscope images of surface (g) and cross section (h) for the Raney Ni catalyst-coated Ni mesh electrode.
The microstructure of the traditional SED was also investigated by characterizing the Raney Ni catalyst coated Ni mesh electrode (CCE) and its interface with the diaphragm. As shown in Fig. 2g, typical CCE exhibits a woven mesh morphology with a rough surface. The cross section SEM image in Fig. 2h reveals that the porous Raney Ni catalyst is distributed around the Ni fibers with a diameter of about 150 μm, and the thickness of the catalyst phase ranges from 100 μm to 150 μm. The surface roughness of the CCE is estimated to be about 50 μm, much bigger than that of the catalyst layer of CCD. Consequently, when the SED is fabricated, an obvious gap is formed between most part of the catalyst in CCE and the diaphragm surface. In particular, only few catalysts were positioned favorably between the Ni fibers of the CCE and the diaphragm for short transport length of OH- ions, while most of the catalysts were positioned rather unfavorably causing long distance for OH- to transport due to poor contact with the diaphragm. Therefore, it is expected that the CCD with catalyst layer directly deposited onto the diaphragm could exhibit much lower ionic resistance than the SED, and at the same time, higher utilization of the catalyst.
Durability of the CCD
The physical stability of the CCD was evaluated by measuring the bond strength of the catalyst layer and the Zirfon 220 diaphragm. The mean adhesive strength measured is in the range of 0.179–0.519 MPa. After pulling apart the catalyst layer from the CCD, it was observed that the ZrO2 nanoparticles were also peeled from the surface of Zirfon 220 diaphragm, indicating strong adhesion of the catalyst layer. This feature is critical for the structural stability of the CCD.
The electrochemical stability of the CCD was also investigated under both dynamic and constant current conditions in the electrolyser according to the recommended method for degradation rate assessment proposed in the EU harmonized protocols28. After 10000 cycles of load current density switches between 0.4 mA cm−2 (close to open circuit) and 2 A cm−2, the voltage degradation rate measured at 2 A cm−2 was 18.5 μV h-1 for the CCD electrolyser, which is about 30% of that for an SED electrolyser (65.6 μV h-1), as shown in Fig. 3a. After more than 1000 h of operation at constant current density between 0.4 A cm−2 and 0.8 A cm−2 (Fig. 3b), the voltage degradation rate was estimated at 2.5 μV h-1 for the CCD electrolyser, which is also lower than that of a comparable SED electrolyser (3.6–4.0 μV h-1). Thus, the CCD exhibits an improved electrochemical durability over the conventional SED. This is a very important feature for CCD’s industrial applications.
a Single electrolysis cell voltage recorded with current density switch between 0.4 mA cm−2 and 2 A cm−2. The duration for each current density hold was set at 30 s. b Single electrolysis cell voltage profiles collected with switched current density operation at 0.4 and 0.8 A cm−2 for more than 1000 h.
The chemical and physical change of the CCD was further analyzed after more than 300 h of operation. In order to demonstrate the reproducibility, two CCD samples with Raney Ni catalysts were tested with constant current density of 0.5 A cm−2 at 80 ± 2 oC. The voltages of both CCDs are between 1.71 V and 1.73 V within the testing duration as shown in Fig. 4a. Occasional voltage rise is due to the addition of water into electrolyte. For one of the CCDs, its polarization curve almost unchanged after the 300 h of operation (Fig. 4b). The fresh and operated CCDs were characterized with the X-ray photoelectron spectroscopy (XPS) and the spectra are displayed in Fig. 4c. Due to the Nafion coverage on the catalyst, very limited signal of Ni was detected for the fresh CCD. After about 8 h of activation, featured peak of Ni (II) 2p was observed at ~855.5 eV. It shifts to higher binding energy by about 0.2 eV for the CCD anode operated by 300 h, indicating the presence of Ni (III) species. For the Nafion binder, the F 1 s peak slightly shifts to lower binding energy after operation, due possibly to the swelling and rearrangement of the polymer chain. The S 2p peak for the CCD anode shifts negatively after activation due to the de-protonization of -SO3H in alkaline electrolyte. For CCD operated by 300 h, the S 2p peak shows no obvious change, indicating relatively stable chemistry of the Nafion side chain. The porosity of the CCD is decreased by more than 50% after the operation of 300 h, which can be attributed to the shrinkage of diaphragm pores due to the pressurization under cell assembling force.
a Single electrolysis cell voltages operated at constant current density of 0.5 A cm−2. b Polarization curves measured after 8 h of activation and 300 h of operation. c X-ray photoelectron spectroscopy signals for fresh and operated catalyst-coated diaphragms. Regions of Ni 2p, F 1 s and S 2p were recorded.
Water electrolysis performance
A substantial performance improvement was achieved for the CCD electrolyser. The polarization curves in Fig. 5a show that the CCD voltage is about 200 mV lower at 2 A cm−2 than that of a traditional SED, which translates into more than 16% increase in efficiency. At voltage of 2 V, usually the maximum allowable operating voltage for an industrial AWE as electrochemical corrosion management dictates so, the CCD reaches a current density as high as 1.8 A cm−2, surpassing the SED (1.3 A cm−2) by more than 40%. As a result, the hydrogen production rate per cell area can be enhanced, leading to a cost reduction in AWEs. What is noteworthy is that, the Raney Ni catalyst loading in a CCD (2 mg cm−2) is less than 10% of that used in an SED (26–30 mg cm−2), while the CCD voltage in the electrochemical reaction control region (current density <0.1 A cm−2) is slightly lower than that in the SED. At voltage of 1.8 V, the calculated mass activity of catalyst with the CCD is about 20-fold that with the SED. Thus, it can be inferred that enlarged electrochemically active surface area has been achieved with the CCD. The electrochemical impedance spectra (EIS) in Fig. 5b reveal that the electrochemical surface area of CCD is 4 times larger, indicating more than forty fold increased catalyst utilization over the SED. Furthermore, the ohmic resistance (Rs) of the CCD cell is more than 30% lower, demonstrating more efficient transport of the OH- ions. As shown in Fig. 1, this is attributed to the shortened travel distance of OH- from cathode to anode, due to the direct contact between the Raney Ni catalyst layer and the diaphragm.
a, b Polarization curve, mass activity (a) and electrochemical impedance spectra (b) for catalyst-coated diaphragm electrolyser compared with separated electrode and diaphragm electrolyser. Raney Ni was used as catalyst with loadings of 2 mg cm−2 and 26–30 mg cm−2 for the catalyst-coated diaphragm and mesh electrode, respectively. All polarization curves are without iR correction. Error bars in the polarization curve of the catalyst-coated diaphragm electrolyser is obtained by evaluation of four samples, and the average voltage at each current density is presented with standard deviation indicated. Mass activity was calculated with the current density at voltage of 1.8 V. The electrochemical impedance spectra were profiled at current density of 0.5 A cm-2. c, d Polarization curves (c) and their Tafel equation fitting (d) for catalyst-coated diaphragms with various catalysts. The catalyst loading was controlled at 2 mg cm-2 for RuO2, Ni-Fe layered double hydroxide (NiFe-LDH) and Raney Ni. For carbon supported Pt (Pt/C), the Pt loading was 0.1 mg cm-2. For Tafel equation fitting, the cell voltage was corrected by subtracting the voltage drop caused by the ohmic resistance (iR).
The advantage of the CCD is further explored by using various types of catalysts, including precious metal and non-precious metal ones. It is worth noting that the preparation of CCD is a physical coating process at sub-100 °C temperatures, a drastically different fabrication process than that used for SED electrode. An SED electrode usually involves sophisticated high temperature thermal spraying9 or electrochemical/chemical deposition methods29,30 to immobilize catalyst onto woven-mesh or porous metal substrate. Furthermore, a variety of powder catalysts are available for the CCD, creating more possibilities for electrode iteration for even better performance in a CCD electrolyser. Typical precious metal catalysts of Pt/C and RuO2 were chosen first for the cathode and anode, respectively, which are considered among the most active catalysts for HER and OER in alkaline media. The obtained CCD denoted as Pt/C(-)|RuO2(+) reaches rather high current density of 1 A cm−2 at 1.8 V and 2 A cm−2 at 2 V with Pt loading of 0.1 mg cm−2 and RuO2 loading of 2 mg cm−2, as shown in Fig. 5c. These results outperform most of the reported anion exchange membrane electrolysers with a current density of 1 A cm−2 at 1.85 V24, and they are comparable to those of an PEME using Nafion 117 membrane with similar thickness to Zirfon 220 diaphragm (185 μm vs. 220 μm)31, indicating the feasibility to develop high-rate AWEs. Subsequently, the anode catalyst was substituted with the Ni-Fe layered double hydroxide (NiFe-LDH), which has been reported as one of the most active non-precious metal catalyst for OER in alkaline media32,33. Indeed, the prepared CCD (denoted as Pt/C(-)|NiFe-LDH(+)) exhibits much lower voltage at a current density below 1 A cm−2, with a voltage drop of more than 50 mV at 0.5 A cm−2. However, worsened performance was observed at a current density above 1 A cm−2, which is ascribed to the poor electronic conductivity of NiFe-LDH34. In addition, the electrochemical stability of NiFe-LDH for OER is yet to be overcome in concentrated KOH electrolyte of AWE due to oxidation and leaching of Ni(II) and Fe(II) species35. Finally, commercial Raney Ni was used as the non-precious metal catalyst for both cathode and anode of the CCD (denoted as RNi(-)|RNi(+)), for the corrosion resistance of Ni in AWE conditions is more than 2 magnitude better than Fe, Pt, and Ru36. With the same catalyst loading at 2 mg cm−2, the performance of RNi(-)|RNi(+) CCD surpassed the one with RuO2 as the anode catalyst (denoted as RNi(-)|RuO2(+)) at a current density below 1.5 A cm−2, inferring that the OER activity of Raney Ni is higher than RuO2. By fitting the polarization curve with Tafel equation (Fig. 5d), it was revealed that the OER exchange current density was enhanced by at least 3 times for Raney Ni. Because of its better electronic conductivity for RuO2, the voltage of RNi(-)|RuO2(+) CCD gradually caught up with the RNi(-)|RNi(+) at a current density near 2 A cm−2. On the other hand, however, a performance gap was found between the RNi(-)|RuO2(+) and Pt/C(-)|RuO2(+) CCDs, implying lower HER activity of Raney Ni than Pt/C. According to the linear-fitted polarization curve in Fig. 5d, it was deduced that the HER exchange current density for Raney Ni was only a third of that of Pt/C. Thus, Raney Ni catalyst exhibits a pretty good OER activity, while the electronic conductivity and the HER activity need to be improved. Further measurements show that the Raney Ni catalyst layer possesses an electronic conductivity at about 1 S m-1, over a magnitude lower than that of Pt/C and even close to that of IrO2 in PEME37,38, which are areas for further improvements. Considering the fact that Raney Ni catalyst is produced from abundant and low-cost raw materials through large scale metallurgy process, higher loading of Raney Ni in CCD is expected to enhance the electrolysis performance with a negligible cost increase in AWEs.
The performance of our CCDs is further compared with recent works and summarized in Table 1. It shows that the CCD route achieves substantially high performance. In particular, for the non-precious metal based AWEs, performance of the CCD is among the best. Therefore, the CCD could be a potential route for the development of high-rate AWEs.
Discussion
In summary, the CCD concept was proposed as an alternative route for the development of high-rate AWEs. Direct coating of catalyst layers on the porous diaphragm has shortened the OH- transport distance and enlarged the electrochemical surface area by over forty fold, demonstrating more than 40% improvement of hydrogen production rate or as high as 16% reduction in energy consumption than conventional SED AWEs. With commercial catalysts and diaphragm, the CCD AWE was capable of delivering a current density as high as 1 A cm−2 at 1.8 V and 2 A cm−2 at 2 V, outperforming most of the anion exchange membrane electrolysers reported. The results are even close to those of the performance of a medium level PEME. Unlike the SED electrode prepared from sophisticated high-temperature thermal spraying or electrochemical/chemical deposition, the fabrication of CCD is a low-temperature physical coating process and can use powder catalysts received. The simplified fabrication of CCD is user friendly to conduct in ether academic labs or industrial batch production lines. This is in turn, expected to dramatically accelerate the technology iteration of CCD AWEs. Preliminary results also show that the CCD structure is relatively solid and durable under harsh alkali and electrochemical conditions of AWEs, meeting the prerequisites for its industrial applications. We believe that the transition from SED to CCD would benefit the development of high-performance AWEs, contributing to large scale production of green hydrogen for future sustainability.
Methods
Materials
Commercial Pt/C (20 wt%, Shanghai Hesen Electric Co., Ltd), RuO2 powder (Beijing InnoChem Science & Technology Co., Ltd), Nafion solution (10 wt%, DuPont), Zirfon 220 diaphragm (AGFA) and Raney Ni coated mesh electrode (Baoshilai New Material Technology Co., Ltd.) were used as received without any further treatment. Analytical grade KOH (85 wt%), Ni(NO3)2•6H2O, Fe(NO3)3 •9H2O and Na2CO3 were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Fresh Raney Ni catalyst (Jiangsu Raney Metal Technology Co., Ltd) was carefully passivized in ambient air for at least 12 h and sieved through mesh to obtain desirable particle size of less than 30 μm. The NiFe-LDH catalyst was prepared by one-step co-precipitation method. Typically, 1 mol of Ni(NO3)2•6H2O and 0.5 mol of Fe(NO3)3 •9H2O were dissolved to obtain aqueous solution and kept at 85 oC. The aqueous solution with 1.75 mol of Na2CO3 was then added under mechanical stirring. After precipitation, the resulting suspension was stirred for another 5 h, and subsequently filtered, washed with deionized water until the pH was measured at 7. Finally, the NiFe-LDH catalyst was collected after dried at 90 oC for 12 h.
Preparation of CCD
Catalyst ink was prepared by mixing the catalyst powder with Nafion solution and water. To obtain homogeneous dispersion, the mixture was sonicated for at least 2 min. The mass ratio of catalyst to dry Nafion ionomer was optimized and controlled at 1: 10 for Pt/C cathode and 1: 1 for RuO2 anode, NiFe-LDH anode, and Raney Ni electrodes. The catalyst ink was blade-coated onto the Zirfon 220 diaphragm and allowed to dry at 80 oC in vacuum for 1 h. The precious metal loading were controlled at 0.1 mg cm−2 for Pt/C cathode and 2 mg cm−2 for RuO2 anode, respectively. For comparison, the catalyst loading of NiFe-LDH anode and Raney Ni electrodes was also controlled at 2 mg cm−2. All the CCDs were hot-pressed at 150 oC and 5 MPa for 5 min before electrolysis performance evaluation.
Physical characterizations
The morphology was observed by a field emission scanning electron microscope (SEM, FEI Nova Nano 450) with energy dispersive X-ray spectroscopy (EDS, Oxford X-maxN 50) mapping and a high resolution transmission electron microscope (HR-TEM, JEM-ARM200F NEOARM). The CCD or CCE samples were cut into 1 cm × 1 cm sections and dipped into a mixture of epoxy resin and hardener (G1, Beijing XXBR Technology Co.,Ltd.) for 20 min. Then, the excess resin mixture was wiped off, and the samples were cured at ambient temperature for at least 24 h. The embedded samples were deposited on mounts and covered with shield plates, and an argon ion beam cross-section polisher (ArBlade 5000, Hitachi High-Tech Co., Ltd.) was used to obtain cross-sections. The three dimensional image of the CCD was taken on a ultra-depth optical microscopes (Leica DM6 M LIBS Wetzlar, Germany) and the surface roughness was calculate by the LeicaMap Premium V9 software. The porosity of Raney Ni catalyst, Zirfon 220 diaphragm and the CCD were measured on a Quantachrome Poremaster GT60 mercury intrusion analyzer. The intrusion and extrusion pressure was set in the range of 0 to 30,000 psi. The adhesion strength measurement of the catalyst layer in CCD was conducted on a LUMiFrac adhesion analyzer according to the standard of ISO 462439. A CCD sample with a diameter of 10 mm was firmly stuck onto the test pedestal with super glue. The rotating speed of the pedestal was gradually increased until the peeling of catalyst layer. The adhesive strength is obtained according to the centrifugal force. The through-plane electronic conductivity of the CCD catalyst layer was measured according to the reference37. The catalyst layer was sprayed onto the aluminum foil and allowed to dried at 80 oC for 3 h. After hot-pressed at 150 oC and 5 MPa for 5 min, the catalyst coated foil was sandwiched between carbon papers to measure the electronic resistance using the four-point probe micro-ohm meter (Rooko, FT-541SJB-341). X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi) was profiled with a 150 W Al Kα X-ray source gun and the spot size is 650 µm. Before test, the operated CCDs were washed with de-ionized water to remove the absorbed KOH.
Water electrolysis performance evaluation
Single cell was used to evaluate the electrolysis performance. The active area was a square with a length of 5 cm each side. Nickle coated stainless steel plate with parallel flow field was used as the end plates. The electrolyte flow area is 5 × 5 cm2, with flow channel width of 1 mm, depth of 1 mm, and rib width of 1 mm. To fabricate the CCD electrolyser, CCD was plated between nickle meshes (thickness of 0.1 mm and mesh number of 200) and then pressed by end plates, with polytetrafluoroethylene gaskets to seal the anode and cathode cavities. For the SED electrolyser, commercial CCEs (catalyst loading of 26 - 30 mg cm−2) were pressed onto both sides of the diaphragm by end plates. The cell assembly pressure for both CCD and SED was controlled at 3 MPa to maintain sufficient electronic conductivity. The 30 wt% KOH aqueous solution was circulated within the cell as electrolyte. After the break-in period of 2 h to obtain stable cell voltage at current density of 0.5 A cm−2, electrolysis polarization curves were measured by tuning the current density from 0.01 to 2 A cm−2. All cells were tested with operation temperature of 80 oC and ambient pressure. In order to demonstrate the reproducibility, four CCD samples with Raney Ni catalyst were evaluated to calculate the voltage deviation at each current density, and the error bar was enclosed for the polarization curve. The EIS was measured with an IVIUMA 08091 electrochemical station at a current density of 0.5 A cm−2, and the disturb current frequency was screened from 10000 to 0.1 Hz. The EIS was fitted using equivalent circuit to obtain internal ohmic resistance (Rs), electrochemical reaction resistance (Rct), and double layer capacitance (Cdl). The electrochemical surface area of the cell is calculated assuming the specific capacitance of 40 µF cm‒2 for Ni based electrocatalysts40.
Data availability
The data supporting the findings of this work are available from the corresponding author upon reasonable request.
References
van Renssen, S. The hydrogen solution? Nat. Clim. Change 10, 799–801 (2020).
Hydrogen for Net-Zero: A Critical Cost-Competitive Energy Vector. https://hydrogencouncil.com/wp-content/uploads/2021/11/Hydrogen-for-Net-Zero.pdf (2021).
Hubert, M. A., King, L. A. & Jaramillo, T. F. Evaluating the case for reduced precious metal catalysts in proton exchange membrane electrolyzers. ACS Energy Lett. 7, 17–23 (2022).
Ouimet, R. J. et al. The role of electrocatalysts in the development of gigawatt-scale PEM electrolyzers. ACS Catal. 12, 6159–6171 (2022).
Schalenbach, M. et al. Acidic or alkaline? Towards a new perspective on the efficiency of water electrolysis. J. Electrochem. Soc. 163, F3197 (2016).
Phillips, R. & Dunnill, C. W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Adv. 6, 100643–100651 (2016).
Ehlers, J. C., Feidenhans’l, A. A., Therkildsen, K. T. & Larrazábal, G. O. Affordable green hydrogen from alkaline water electrolysis: key research needs from an industrial perspective. ACS Energy Lett. 8, 1502–1509 (2023).
Hodges, A. et al. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 13, 1304 (2022).
Razmjooei, F. et al. Improving plasma sprayed Raney-type nickel–molybdenum electrodes towards high-performance hydrogen evolution in alkaline medium. Sci. Rep. 10, 10948 (2020).
Lee, S. H. & Rasaiah, J. C. Proton transfer and the mobilities of the H+ and OH− ions from studies of a dissociating model for water. J. Chem. Phys. 135, 124505 (2011).
Duso, A. B. & Chen, D. D. Y. Proton and hydroxide ion mobility in capillary electrophoresis. Anal. Chem. 74, 2938–2942 (2002).
Liu, L., Chen, W. & Li, Y. An overview of the proton conductivity of nafion membranes through a statistical analysis. J. Membr. Sci. 504, 1–9 (2016).
Lee, H. I. et al. Advanced Zirfon-type porous separator for a high-rate alkaline electrolyser operating in a dynamic mode. J. Membr. Sci. 616, 118541 (2020).
Xie, X. et al. Oxygen evolution reaction in alkaline environment: material challenges and solutions. Adv. Funct. Mater. 32, 2110036 (2022).
Xia, Y., Cheng, H., He, H. & Wei, W. Efficiency and consistency enhancement for alkaline electrolyzers driven by renewable energy sources. Commun. Eng. 2, 22 (2023).
Gu, J. et al. Experimental studies on dynamic performance of 250-kW alkaline electrolytic system. J. Power Sources 592, 233920 (2024).
Kuleshov, N. V. et al. Development and performances of a 0.5 kW high-pressure alkaline water electrolyser. Int. J. Hydrog. Energy 44, 29441–29449 (2019).
Stiber, S. et al. Porous transport layers for proton exchange membrane electrolysis under extreme conditions of current density, temperature, and pressure. Adv. Energy Mater. 11, 2100630 (2021).
Krishnan, S. et al. Present and future cost of alkaline and PEM electrolyser stacks. Int. J. Hydrog. Energy 48, 32313–32330 (2023).
de Groot, M. T. & Vreman, A. W. Ohmic resistance in zero gap alkaline electrolysis with a Zirfon diaphragm. Electrochim. Acta 369, 137684 (2021).
Haverkort, J. W. & Rajaei, H. Voltage losses in zero-gap alkaline water electrolysis. J. Power Sources 497, 229864 (2021).
Nuttall, L. J. Conceptual design of large scale water electrolysis plant using solid polymer electrolyte technology. Int. J. Hydrog. Energy 2, 395–403 (1977).
Smolinka, T., Bergmann, H., Garche, J. & Kusnezoff, M. The history of water electrolysis from its beginnings to the present. in Hydrogen production by water electrolysis (Elsevier, 2022).
Du, N. et al. Anion-exchange membrane water electrolyzers. Chem. Rev. 122, 11830–11895 (2022).
Karacan, C. et al. Fabrication of high performing and durable nickel-based catalyst coated diaphragms for alkaline water electrolyzers. J. Electrochem. Soc. 169, 054502 (2022).
Girod, R., Lazaridis, T., Gasteiger, H. A. & Tileli, V. Three-dimensional nanoimaging of fuel cell catalyst layers. Nat. Catal. 6, 383–391 (2023).
Lee, J. H. et al. Dispersion-solvent control of ionomer aggregation in a polymer electrolyte membrane fuel cell. Sci. Rep. 8, 10739 (2018).
Tsotridis, G. & Pilenga, A. EU Harmanoised Protocols for Testing of Low Temperature Water Electrolysers. (2021).
Zuo, Y. et al. High-performance alkaline water electrolyzers based on Ru-perturbed Cu nanoplatelets cathode. Nat. Commun. 14, 4680 (2023).
Li, L. et al. High-rate alkaline water electrolysis at industrially relevant conditions enabled by superaerophobic electrode assembly. Adv. Sci. 10, 2206180 (2023).
Pham, C. V., Escalera-López, D., Mayrhofer, K., Cherevko, S. & Thiele, S. Essentials of high performance water electrolyzers – from catalyst layer materials to electrode engineering. Adv. Energy Mater. 11, 2101998 (2021).
Dionigi, F. et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 11, 2522 (2020).
Magnier, L. et al. Fe–Ni-based alloys as highly active and low-cost oxygen evolution reaction catalyst in alkaline media. Nat. Mater. https://doi.org/10.1038/s41563-023-01744-5 (2024).
Liao, Y. et al. Lattice distortion induced Ce-doped NiFe-LDH for efficient oxygen evolution. Chem. Eng. J. 464, 142669 (2023).
Tyndall, D. et al. Demonstrating the source of inherent instability in NiFe LDH-based OER electrocatalysts. J. Mater. Chem. A 11, 4067–4077 (2023).
Speck, F. Degradation Mechanisms of Electrocatalysts in Alkaline Media. (Friedrich-Alexander-Universität Erlangen-Nürnberg, 2020).
Ahadi, M., Tam, M., Stumper, J. & Bahrami, M. Electronic conductivity of catalyst layers of polymer electrolyte membrane fuel cells: through-plane vs. in-plane. Int. J. Hydrog. Energy 44, 3603–3614 (2019).
Padgett, E. et al. Catalyst layer resistance and utilization in PEM electrolysis. J. Electrochem. Soc. 170, 084512 (2023).
ISO 4624: 2023 Paints and varnishes — Pull-off test for adhesion. (2023).
Cossar, E., Houache, M. S. E., Zhang, Z. & Baranova, E. A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 870, 114246 (2020).
Ali, M. F. et al. Zirconia toughened alumina-based separator membrane for advanced alkaline water electrolyzer. Polymers 14, 1173 (2022).
Lee, J. W. et al. Cellulose nanocrystals–blended zirconia/polysulfone composite separator for alkaline electrolyzer at low electrolyte contents. Chem. Eng. J. 428, 131149 (2022).
Colli, A. N., Girault, H. H. & Battistel, A. Non-precious electrodes for practical alkaline water electrolysis. Materials 12, 1336 (2019).
Yuan, X., Yan, T., Liu, Z. & Kang, P. Highly efficient alkaline water electrolysis using alkanolamine-functionalized zirconia-blended separators. ACS Sustain. Chem. Eng. 11, 4269–4278 (2023).
Acknowledgements
We acknowledge financial support from National Key Research and Development Program of China (No. 2023YFB4005000) and CHN Energy Investment Group Co., LTD (No. GJNY−23-95).
Author information
Authors and Affiliations
Contributions
Z.X. designed the research and wrote the manuscript. Y.L., X.C., and Y.S. conducted the experiments. Y.W, J.W., and W.L. performed the characterizations. G.H. conceptualized the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Engineering thanks Kathy Ayers, Sergio Bakovic and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: SungHoon Hur and Ros Daw, Saleem Denholme, Miranda Vinay.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Z., Liu, Y., Cheng, X. et al. A catalyst-coated diaphragm assembly to improve the performance and energy efficiency of alkaline water electrolysers. Commun Eng 4, 9 (2025). https://doi.org/10.1038/s44172-025-00344-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44172-025-00344-2