Abstract
There are repeated calls for remote sensing observations to produce accessible data products that improve our understanding and conservation of biodiversity. The Biodiversity Survey of the Cape (BioSCape) addresses this need by integrating field, airborne, satellite, and modeling datasets to advance the limits of global remote sensing of biodiversity. Over six weeks, an international team of ~150 scientists collected data across terrestrial, marine, and freshwater ecosystems in South Africa. In situ biodiversity observations of plant and animal communities, estuaries, kelp, and plankton were made using traditional field methods as well as novel approaches like environmental DNA and acoustic surveys. Biodiversity observations were accompanied by an unprecedented combination of airborne imaging spectroscopy and lidar measurements acquired across 45,000 km2. Here, we review how the approaches applied in BioSCape will help us measure and monitor biodiversity at scale and the role of remote sensing in accomplishing this.
Similar content being viewed by others
Introduction
With over one million species’ existence threatened, it is widely recognized that we are in the midst of a sixth mass extinction1. Safeguarding biodiversity demands that the “best available data, information and knowledge, are accessible to decision makers, practitioners and the public”2. Creating global biodiversity data products is complex; biogeographic zonation and independent evolutionary histories combined with climatic and environmental variation make biodiversity intrinsically site-specific and one-size-fits-all solutions inappropriate. High-fidelity biodiversity data products require local knowledge and field data, which can be labour intensive and expensive to collect. Recently, technological advancements in survey techniques have increased field data coverage, but spatio-temporal gaps in these measurements persist3. Next-generation remote sensing technology and models can help fill gaps in field data and produce integrated multi-scalar data sets that accelerate biodiversity product generation and improve tracking of progress towards conservation targets4,5,6,7. Due to the novelty of such approaches, there is an urgent need to better understand the potential and limitations of integrated field, remote sensing, and modeling datasets for measuring and monitoring biodiversity globally8.
Integrated multi-scalar datasets are useful for uncovering the ecological processes responsible for observed patterns in species abundance and distribution and their change in time and space9. For example, integrated airborne imaging and field spectroscopy datasets allow the analysis of biodiversity and its relationship to ecosystem function continuously across communities and environmental gradients10,11,12,13. The addition of three-dimensional structural information to these datasets further enhances their ecological applications14. Looking forward, integrated datasets will increasingly include measurements from novel field survey approaches, such as autonomous sound recordings and environmental DNA (eDNA), often combined with citizen science observations15,16. Integrated datasets will also more prominently feature next-generation satellite products like light detection and ranging (LiDAR, herein lidar) and other structural data as well as ultraviolet (UV), visible to shortwave infrared (VSWIR), and thermal infrared (TIR) data from imaging spectrometers, as these expand in area covered, spatial resolution, and temporal latency17,18,19.
Quantifying the opportunities and shortcomings of Open Access integrated datasets to conserve biodiversity has never been more important. Addressing this need motivated the US’s National Aeronautics and Space Administration’s (NASA) first field campaign focused on biodiversity - the Biodiversity Survey of the Cape (BioSCape)—which took place in South Africa in late 2023. Here, we introduce BioSCape, discuss the motivations for choosing South Africa, and review how the campaign advances remote sensing of biodiversity.
The Biodiversity Survey of the Cape
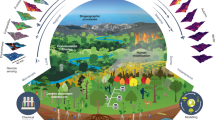
BioSCape pairs diverse field measurements made on land and in water with remotely sensed airborne and satellite observations to better understand the structure, function, and composition of ecosystems and how and why they are changing in time and space. BioSCape is organized around three research themes (Fig. 1):
-
1.
Shifting community composition;
-
2.
Ecosystem disturbance, resilience, and recovery; and
-
3.
Ecosystem function and nature’s contributions to people.
Airborne data were acquired contemporaneously with a variety of field measurements of biodiversity. These data are processed through models and algorithms to produce products that inform our understanding of functional, taxonomic, phylogenetic, and spectral dimensions of biodiversity. Applying these biodiversity data products will help inform the measuring and management of biodiversity resources and nature’s contribution to people (Artwork created by J.Silver under contract with BioSCape).
Field data were collected by 19 individually funded research projects, each with separate but coordinated objectives (see Expected Contributions). BioSCape’s field data set is unique in its diversity and scope—containing novel and traditional field survey measurements in many types of aquatic and terrestrial ecosystems and across multiple environmental gradients. Terrestrial field observations included surveys of more than 600 vegetation plots in protected areas across environmental gradients and additional surveys done in estuarine ecosystems and in landscapes heavily invaded by invasive alien plants. Terrestrial observations also included multi-season sampling of water, soil, and sediment environmental DNA (eDNA) at 36 sites across two watersheds and audio recordings of birds and frogs at more than 500 sites across the region. In the aquatic realm, observations included kelp forest composition and physiological condition at 15 sites as well as biomass, abundance, and taxonomic identification of phyto- and zoo- plankton functional types in four marine bays and four freshwater bodies.
Contemporaneous airborne data were acquired using six sensors aboard two NASA and one South African Environmental Observation Network (SAEON) aircraft in October and November 2023. UV and VSWIR measurements were collected by the Airborne Visible/Infrared Imaging Spectrometer-Next Generation (AVIRIS-NG)20 and the Portable Remote Imaging Spectrometer (PRISM)21 integrated onto a NASA Gulfstream III aircraft, while the Hyperspectral Thermal Emission Spectrometer (HyTES)22 and the Land, Vegetation, and Ice Sensor (LVIS)23 were integrated onto a NASA Gulfstream V to collect TIR and full-waveform lidar measurements respectively. At select coastal sites, additional discrete return lidar data (~10 points per metre) were acquired at by an ELMAP-V instrument and high-resolution colour imagery (~13 cm pixels) were acquired by a 46MP Nikon D850, both integrated onto the SAEON Airborne Remote Sensing Platform. BioSCape’s airborne dataset is unique in its spectral and spatial resolution, covering much of the electromagnetic spectrum at ~2–15 m ground sample distance (Fig. 2). BioSCape’s imaging spectrometers provide data in nearly 1000 well-resolved bands from 350 nm (UV) to 12,000 nm (TIR) at a resolution of 3.5 nm in the UV range to 5 nm in the SWIR range and 17.6 nm in the TIR range. The addition of coincident full-waveform large-footprint lidar acquisitions (1064 nm-wavelength laser) as well as discrete return lidar (1030 nm laser pulse) complement the optical measurements. Accompanying the airborne measurements are numerous satellite acquisitions, including 240 scenes from EMIT24, 101 from ECOSTRESS25, 1,051 from Sentinel-226, 168 from Landsat27, and historical GEDI coverage28. BioSCape thus represents one of the few combined field, airborne, and orbital spectral and structural datasets available. While the airborne data is collected once off, it will serve as a powerful resource to explore the boundaries of remote sensing for biodiversity measurement across diverse aquatic and terrestrial ecosystems, and through time via links with ongoing satellite acquisitions29.
This figure summarizes the key spectral characteristics of BioSCape's three imaging spectrometers and compares them with commonly used instruments with free and open access data policies, and anticipated future missions. A Data were collected across the electromagnetic spectrum, from 350 nm (UV) to 2500 nm (SWIR) using AVIRIS-NG and PRISM and B from 7500 nm to 12 000 nm (TIR) using HyTES, at a resolution of 3.5 nm in the UV range to 5 nm in the SWIR range and 17 nm in the TIR range. C Full waveform airborne lidar data were also acquired using the LVIS instrument. LVIS measures the full distribution of returned laser pulses to the active sensor, capturing more information about topography and vegetation structure than standard discrete return airborne lidar sensors, which typically capture 1-4 discrete points from a single laser pulse. (Artwork in 2c created by A. Ibrahim and J.Flora under contract with BioSCape).
Why South Africa?
BioSCape is focused on the Greater Cape Floristic Region (GCFR) of South Africa. The GCFR is home to astonishing levels of biodiversity, wicked conservation challenges30,31, and a well-developed and progressive biodiversity research and conservation community32,33, together providing fertile ground for local impact and global lessons.
A megadiverse mesocosm of global challenges
The GCFR includes the intersections of eight terrestrial and six marine biomes and is known for its extreme topographic and environmental heterogeneity (Fig. 3). It is home to two of the world’s richest biodiversity hotspots, containing some 11,500 species of vascular plants, of which 78% are endemic to the region34,35. The GCFR is also where the cold Benguela and the warm Agulhas Currents meet, leading to regional peaks in marine biodiversity and some of the highest levels of marine endemism globally, with up to a third of about 13,000 marine species being endemic36,37,38. The GCFR’s freshwater aquatic systems also display high amounts of endemism in their fish, frogs, and invertebrate fauna and are listed among the World’s 200 Significant Ecoregions39.
A Terrestrial biomes and marine bioregions of the BioSCape study area (the Greater Cape Floristic Region) in the South-Western corner of South Africa and (B–D) Airborne data coverage by four of the airborne instruments over the study area. (B–D are screengrabs from the BioSCape Data Portal, popo.jpl.nasa.gov/mmgis-aviris/?mission = BIOSCAPE).
Like many regions worldwide, the GCFR needs to support human development while conserving its biodiversity in the face of climate change. The region is increasingly drought-prone and the oceans are experiencing rapid warming of offshore ocean currents and upwelling intensification with further increases in ocean temperature, ocean acidity, and storm frequency predicted40,41,42. Climate change effects are compounded by significant and somewhat stochastic community reorganization after disturbance by fire or hydrological shocks and by pressure from dense human populations43,44. As a result, the GCFR has the second highest documented number of vascular plant extinctions in the world, over half of marine and coastal ecosystems are threatened, and nearly half of marine fisheries are over-exploited or collapsed38,45.
A region of challenges and opportunities
The megadiverse GCFR presents a challenge for remote sensing of biodiversity. On land, the high plant richness is the result of the radiation of a limited number of lineages, which, together with convergent evolution of similar traits among different lineages, has resulted in large numbers of species that are difficult to distinguish46,47. From a remote sensing perspective, this is aggravated by shrubs being small enough that multiple individuals, and possibly multiple taxonomic groups, are often present in a single pixel. The extreme topography of the region also makes post-processing of remote sensing data difficult and can compromise data quality. In aquatic environments, the optical characteristics and complexity of freshwater and marine environments vary wildly based on nutrient availability and excess. These issues, combined with variations in sediment loads and dissolved organic carbon concentrations that are driven in part by highly localized vegetation and land cover characteristics and land-to-sea biogeochemical transformations, make the applicability of globally-calibrated algorithms challenging for the region48,49,50. A lack of aquatic bio-optical datasets in the global south confounds these challenges. In selecting the GCFR, we hope to push the best available technology and theory up to and beyond its current limits, thereby highlighting areas for future research that will result in even broader utility.
Local impact and global lessons
South Africa is a well-known early adopter of systematic conservation planning and has been relatively successful in operationalizing scientific findings into government policy and decision-making51. The local scientific community is deeply engaged with research that speaks to the information needs of stakeholders, and BioSCape has strongly emphasized incorporating local knowledge from its inception33,52. We expect that BioSCape’s data products will feed into the GCFR’s established pathways from science to application. In doing so, we hope that BioSCape can help set an example of science diplomacy and research for good.
Expected Contributions
All BioSCape data products will be Open Access, and will be accessible through a cloud computing environment throughout the analytical stage of the project. In addition to the regularly delivered Level 1 and 2 airborne data products, BioSCape is also producing Level 3 orthomosaics of surface reflectance from AVIRIS-NG and PRISM, relative heights from LVIS, and possibly surface temperature and emissivity from HyTES. These orthomosaics will be co-registered to a common grid with a common spatial resolution (5 × 5 m, with select regions at 2 × 2 m). This level of data harmonization is unprecedented for an Open Access multi-sensor airborne campaign and we hope will set a new standard for data accessibility53. BioSCape’s data analysis workflows, including airborne data harmonization, will also largely be open source and made available with publications and/or on public GitHub repositories versioned with a DOI, and all data will be made available through the Oak Ridge National Laboratory Distributed Data Archiving Centre54. This will increase interoperability of data products and the reproducibility of the science. In this section, we summarize how the campaign is using data optimized for usability to test the limits and potential of remote sensing for global biodiversity applications.
The spatial resolution of BioSCape pixels, while finer than any spaceborne spectroscopic measurements to date, are typically coarser than the individual organisms being studied and result in pixels with mixed spectral signatures. While one can often identify the endmembers (species or cover types) contributing to the mixed signal using spectral unmixing (e.g.55, another approach is to look at the diversity of spectral signatures across a window of pixels. Spectral diversity has been shown to predict functional and phylogenetic diversity as well as ecosystem function, although there are limitations to this approach56,57,58. Methods like Intrinsic Dimensionality (ID), that allow us to estimate spectral diversity without training data, are some of the few viable approaches to map biodiversity at a global scale using comparable metrics across geographic regions59,60,61,62. Measures of spectral beta diversity can be combined with statistical techniques such as Generalized Dissimilarity Modeling or Spatial Generalized Dissimilarity Mixed Modeling to explore drivers of spectral turnover or to estimate spectral gamma diversity63,64. ID has been used to estimate diversity in marine phytoplankton communities and tropical forest and desert landscapes, but it has never been extensively calibrated with field observations, with multiple sensors, and across environmental or diversity gradients61,65. BioSCape represents the largest application of ID to field data and will help us delineate what this promising method can do for remote sensing of biodiversity at the global scale.
Spectral signatures are inherently a combination of the underlying structure and chemistry of the system; put another way, functional traits define the spectroscopy of plants. Pixels with similar spectral signatures, so called “spectral species”66,67, and their associated functional traits can be mapped across a landscape68. In land plants, certain functional traits can be mapped using empirical relationships derived between field-measured trait values and associated airborne spectral reflectance values69,70. This approach works for single sites and can work for regions, but it struggles to scale further since the calibration relationships can be site-, and sensor-, or date- specific69. The challenge of parameterizing spectra-trait relationships that are applicable globally is further exacerbated by the geographic bias in imaging spectroscopy data acquisition. To date, very little imaging spectroscopy has been acquired on the African continent. BioSCape provides a unique opportunity to test the potential impact of this geographic bias and will compare similar ecosystems across continents and assess both the difference in calibration equations and the accuracy of these equations to map functional traits from remotely sensed imagery71. The BioSCape AVIRIS-NG acquisitions, the first from this sensor on the African continent, are well placed to be compared to the extensive acquisitions along the West Coast of the United States - which has, in parts, a similar climate and vegetation biome to the GCFR. Once functional traits have been mapped, they can be clustered and examined with environmental variables and structural data to better understand the drivers of biodiversity and inform conservation decisions72.
Leaf spectra have been demonstrated to correlate with plant phylogenetic history73. Phylogenetic diversity does not perfectly align with functional diversity due to trait convergence, which causes different lineages living in similar environments to develop similar functional traits. When evaluated with spectral and functional diversity, phylogenetic diversity can provide deep insight into the drivers of functional trait variation across abiotic gradients and spatial scales74. The links between spectral, functional, and phylogenetic diversity have been explored for the BioSCape study region using leaf-level data56 but can now be tested using airborne and satellite remote sensing data. The astonishing levels of taxonomic diversity and yet surprisingly low variation in physiognomy in this biodiversity hotspot will test the limits of these methods47.
In aquatic environments, remote sensing of biodiversity is challenging as vegetation and phytoplankton are often partially or entirely submerged in water, interfering with their spectral signature. In coastal and inland waters, high concentrations of suspended sediments or coloured dissolved organic matter (CDOM) also make it difficult to detect and discriminate between different plant and phytoplankton functional types75,76. Adding to this challenge, the water leaving signal is a very small component of the overall signal received by the sensor (usually less than 10%)77. To resolve phytoplankton and plant functional types, a sensor must have a high signal-to-noise ratio to detect small changes to water-leaving radiances and distinguish these from the water and atmospheric radiance measurements. Additionally, the sensor needs to capture high spatial resolution data, since freshwater floating plants and marine kelp may be limited in their extent, and data must be collected with relatively low solar zenith angles to avoid sunglint from impeding the signal78,79,80. BioSCape’s data over coastal and inland waters has a high spatial (~1–15 m) and spectral resolution and excellent signal-to-noise ratios, with PRISM having a ratio of 500 or more to one at 450 nm. By coupling PRISM and AVIRIS-NG on the same platform, BioSCape made simultaneous spectral measurements across the UV to the SWIR, which may enable improvements in corrections for sunglint and aerosols to enhance remote sensing detection of aquatic biodiversity. When coupled with the near-simultaneous acquisition of thermal imaging spectroscopy from HyTES, this is the first time that such detailed radiance measurements have been collected by multiple spectrometers concurrently with field measurements at the confluence of major oceanic currents and over freshwater bodies.
Detecting change in biodiversity is difficult, as any departure from expected radiance or reflectance values needs to be attributable to a change in community composition or ecosystem function rather than a background change in the atmosphere or water column values. One can use first-principle physical laws to address this attribution challenge by using radiative transfer models (RTMs) to generate synthetic background or baseline measurements of a system81,82. RTMs’ synthetic measurements can be compared to measured radiance and reflectance values to help disentangle disturbance signals from background noise. RTMs are especially useful for remote sensing of phytoplankton communities, where differentiating signals from the water column and the target communities is difficult83. RTMs are also useful in terrestrial plant communities where there is high natural variability through space and time and thus departures from the “natural” state of the ecosystem can be hard to detect unless one has a suitable model of the expected signal of a “natural” state84,85. In inland and coastal waters, BioSCape’s innovative combination of sensors will facilitate development and testing of new machine learning algorithms, making an essential contribution to water quality measurement and monitoring86. On land, BioSCape will use 3D RTMs to develop synthetic reflectance datasets for several different vegetation types that will be compared to terrestrial and airborne lidar and airborne imaging spectroscopy datasets87. These next-generation RTMs can assist in detecting alien plant invasions and abnormal post-fire vegetation recovery trajectories, as well as provide baseline datasets for various functional and structural trait measurements.
In the GCFR and other Mediterranean ecosystems, one of the most widespread changes in community composition and threats to biodiversity is the increase in the abundance of invasive alien plants88. Invasive alien plants decrease biodiversity by replacing indigenous plant species and disrupt the normal fire regime by altering fuel loads and fire severity and frequency89,90. Invasive alien trees in the region also alter ecosystem function by dramatically increasing water use and evapotranspiration while decreasing runoff and groundwater recharge91,92,93. Groundwater-dependent ecosystems provide many contributions to people, including ensuring water quality, and their sensitivity to invasive alien trees and groundwater abstraction is largely unknown as the linkages between surface and groundwater are highly complex94. BioSCape will combine spectral information with structural information to quantify and map alien invasive trees, flammable fuel loads, and water use efficiency across various invaded landscapes as well as groundwater dependent ecosystems in the region.
Changes in aquatic community composition can affect ecosystem function and services. For example, phytoplankton functional type and abundance are key determinants of water quality, kelp forests support healthy fish populations, remove nitrogen from the water column and may act as blue carbon stores, while estuaries play a role in coastal protection and maintenance of fisheries95,96,97. BioSCape will develop and apply algorithms for airborne spectroscopy to map phytoplankton functional types based on their accessory pigments and to map kelp forest extent and physiological condition. By developing these algorithms for multiple marine and, where relevant, inland water bodies across anthropogenic and environmental gradients, BioSCape will contribute towards harmful algal bloom and kelp forest monitoring efforts. Additionally, BioSCape will map estuarine biodiversity variables to make predictions about how these systems’ contributions to people may change with changes in climate, especially sea level rise. All of these research objectives will have global applicability, but are especially important to address in water scarce regions with little redundancy in drinking water supply and in places with economically important fishing and aqua- and agri- culture industries, as is the case in the GCFR98,99.
Understanding the complex links between biodiversity, ecosystem function, and nature’s contribution to people requires not only different types of remote sensing data but also an abundance of high-quality field measurements. Technological advancements in field methods have changed the type and increased the amount of low-cost high-coverage biodiversity information we can collect. The novelty of these field biodiversity measurements means they have not yet been reliably related to remotely sensing biodiversity information. For example, multi-locus metabarcoding of eDNA from soils, sediment, and water can extend species identification to microorganisms and non-vascular plants, in addition to the vascular plants and vertebrates captured in traditional field surveys5,15. These molecular methods can facilitate new insights into the organization of ecological communities - for instance, eDNA samples collected in rivers can provide an integrated field-based measure of biodiversity across the entire watershed and can be better than traditional surveys at detecting small, rare, or otherwise cryptic species100,101. Genetic composition is the only Essential Biodiversity Variable class “not yet measurable from space”7. To address this gap, BioSCape will correlate ground-based eDNA estimates of allelic diversity with remotely sensed diversity metrics across multiple watersheds and along a gradient of human influence. Such an approach has been successfully trialed in California5, and BioSCape hopes to advance this field significantly. Like eDNA, autonomous recording units deployed across a landscape allow us to gather biodiversity information about birds, frogs, and other potential indicator species over large areas quickly and at relatively low cost16,102,103,104,105. BioSCape will aim to link acoustic diversity with plant and structural diversity determined from airborne spectroscopy and lidar data. Both eDNA and acoustic diversity provide novel biodiversity information that, when paired with remote sensing data, has great potential to improve the accuracy of biodiversity measurement at regional to global scales.
Conclusion
Addressing biodiversity loss is a global priority and there is a clear need to improve our ability to map and monitor change. Due to its complexity, the GCFR represents one of the most challenging environments for remote sensing of biodiversity, but also presents myriad opportunities. BioSCape’s findings will advance our understanding of biodiversity in South Africa and have far-reaching implications for global biodiversity science, inclusive international research projects, data-driven conservation, and effective ecosystem management. All BioSCape data are Open Access and will be made available through the Oak Ridge National Laboratory Distributed Data Archiving Centre54. The project’s innovative methods and insights will help define the potential and the limits of biodiversity measurement and monitoring using remote sensing. In doing so, we hope BioSCape can guide the development of appropriate biodiversity proxies that can be observed from space, ultimately contributing to preserving our planet’s rich biodiversity.
Data Availability
No datasets were generated or analysed during the current study.
References
The Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), Bonn, 2019).
Conference of the Parties to the Convention on Biological Diversity. 15/5. Monitoring framework for the Kunming-Montreal Global Biodiversity Framework. (2022).
Oliver, R. Y. et al. Camera trapping expands the view into global biodiversity and its change. Philos. Trans. R. Soc. B 378, 20220232 (2023).
Leaper, R., Dunstan, P. K., Foster, S. D., Barrett, N. J. & Edgar, G. J. Comparing large‐scale bioregions and fine‐scale community‐level biodiversity predictions from subtidal rocky reefs across south‐eastern Australia. J. Appl. Ecol. 49, 851–860 (2012).
Lin, M. et al. Landscape analyses using eDNA metabarcoding and Earth observation predict community biodiversity in California. Ecological Applications 31, (2021).
Rhodes, C. J., Henrys, P., Siriwardena, G. M., Whittingham, M. J. & Norton, L. R. The relative value of field survey and remote sensing for biodiversity assessment. Methods Ecol. Evol. 6, 772–781 (2015).
Skidmore, A. K. et al. Priority list of biodiversity metrics to observe from space. Nat. Ecol. Evol. 5, 896–906 (2021).
Cavender-Bares, J. et al. Integrating remote sensing with ecology and evolution to advance biodiversity conservation. Nat. Ecol. Evol. 6, 506–519 (2022).
Chase, J. M. & Knight, T. M. Scale‐dependent effect sizes of ecological drivers on biodiversity: why standardised sampling is not enough. Ecol. Lett. 16, 17–26 (2013).
Asner, G. P. & Martin, R. E. Spectranomics: Emerging science and conservation opportunities at the interface of biodiversity and remote sensing. Glob. Ecol. Conserv. 8, 212–219 (2016).
Durán, S. M. et al. Informing trait-based ecology by assessing remotely sensed functional diversity across a broad tropical temperature gradient. Sci. Adv. 5, eaaw8114 (2019).
Ustin, S. L., Roberts, D. A., Gamon, J. A., Asner, G. P. & Green, R. O. Using Imaging Spectroscopy to Study Ecosystem Processes and Properties. BioScience 54, 523 (2004).
Ustin, S. L. & Middleton, E. M. Current and near-term advances in Earth observation for ecological applications. Ecol. Process 10, 1 (2021).
LaRue, E. A. et al. A theoretical framework for the ecological role of three‐dimensional structural diversity. Front. Ecol. Environ. 21, 4–13 (2023).
Meyer, R. S. et al. The CALeDNA program: Citizen scientists and researchers inventory California’s biodiversity. Calif. Agr. 75, 20–32 (2021).
Snyder, R. et al. The Soundscapes to Landscapes Project: Development of a Bioacoustics-Based Monitoring Workflow with Multiple Citizen Scientist Contributions. CSTP 7, 24 (2022).
Albinet, C. et al. A Joint ESA-NASA Multi-mission Algorithm and Analysis Platform (MAAP) for Biomass, NISAR, and GEDI. Surv. Geophys 40, 1017–1027 (2019).
Gorman, E. et al. The NASA Plankton, Aerosol, Cloud, ocean Ecosystem (PACE) mission: an emerging era of global, hyperspectral Earth system remote sensing. in Sensors, Systems, and Next-Generation Satellites XXIII (eds. Neeck, S. P., Kimura, T. & Martimort, P.) 15. https://doi.org/10.1117/12.2537146 (SPIE, Strasbourg, France, 2019).
Stavros, E. N. et al. Designing an Observing System to Study the Surface Biology and Geology (SBG) of the Earth in the 2020s. JGR Biogeosciences 128, e2021JG006471 (2023).
Chapman, J. W. et al. Spectral and Radiometric Calibration of the Next Generation Airborne Visible Infrared Spectrometer (AVIRIS-NG). Remote Sens. 11, 2129 (2019).
Mouroulis, P. et al. Design of an Airborne Portable Remote Imaging Spectrometer (PRISM) for the Coastal Ocean. (2014).
Hook, S. J., Johnson, W. R. & Abrams, M. J. NASA’s Hyperspectral Thermal Emission Spectrometer (HyTES). in Thermal Infrared Remote Sensing (eds. Kuenzer, C. & Dech, S.) 17 93–115 (Springer Netherlands, Dordrecht, 2013).
Blair, J. B., Rabine, D. L. & Hofton, M. A. The Laser Vegetation Imaging Sensor: a medium-altitude, digitisation-only, airborne laser altimeter for mapping vegetation and topography. ISPRS J. Photogramm. Remote Sens. 54, 115–122 (1999).
Green, R. O. et al. The Earth Surface Mineral Dust Source Investigation: An Earth Science Imaging Spectroscopy Mission. in 2020 IEEE Aerospace Conference 1–15. https://doi.org/10.1109/AERO47225.2020.9172731 (IEEE, Big Sky, MT, USA, 2020).
Hulley, G., Hook, S., Fisher, J. & Lee, C. ECOSTRESS, A NASA Earth-Ventures Instrument for studying links between the water cycle and plant health over the diurnal cycle. in 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS) 5494–5496. https://doi.org/10.1109/IGARSS.2017.8128248 (IEEE, Fort Worth, TX, 2017).
Fernandez, V., Martimort, P., Spoto, F., Sy, O. & Laberinti, P. Overview of Sentinel-2. in (eds. Meynart, R., Neeck, S. P. & Shimoda, H.) 88890K. https://doi.org/10.1117/12.2028755 (Dresden, Germany, 2013).
Masek, J. G. et al. Landsat 9: Empowering open science and applications through continuity. Remote Sens. Environ. 248, 111968 (2020).
Dubayah, R. et al. The Global Ecosystem Dynamics Investigation: High-resolution laser ranging of the Earth’s forests and topography. Sci. Remote Sens. 1, 100002 (2020).
Dierssen, H. M. et al. Synergies Between NASA’s Hyperspectral Aquatic Missions PACE, GLIMR, and SBG: Opportunities for New Science and Applications. JGR Biogeosciences 128, e2023JG007574 (2023).
Rittel, H. W. J. & Webber, M. M. Dilemmas in a General Theory of Planning. Policy Sci. 4, 155–169 (1973).
Van Wilgen, B. W. et al. Ecological research and conservation management in the Cape Floristic Region between 1945 and 2015: History, current understanding and future challenges. Trans. R. Soc. South Afr. 71, 207–303 (2016).
Balmford, A. Conservation planning in the real world: South Africa shows the way. Trends Ecol. Evolution 18, 435–438 (2003).
Gelderblom, C. & Wood, J. The Fynbos Forum: Its Impact and History. (2018).
Manning, J. & Goldblatt, P. Plants of the Greater Cape Floristic Region. 1: The Core Cape flora. Plants of the Greater Cape Floristic Region. 1: The Core Cape flora. (2012).
Snijman, D. A. Plants of the Greater Cape Floristic Region. 2: The Extra Cape flora. Plants of the Greater Cape Floristic Region. 2: The Extra Cape flora. (2013).
Bolton, J. J. Seaweed Systematics and Diversity in South Africa: An Historical Account. Trans. R. Soc. South Afr. 54, 167–177 (1999).
Griffiths, C. L., Robinson, T. B., Lange, L. & Mead, A. Marine Biodiversity in South Africa: An Evaluation of Current States of Knowledge. PLoS ONE 5, e12008 (2010).
Harris, L. R., Sink, K., Skowno, A. L. & Van Niekerk, L. South African National Biodiversity Assessment 2018 Technical Report Volume 5: Coast. http://hdl.handle.net/20.500.12143/6374 (2019).
de Moor, F. C. & Day, J. A. Aquatic biodiversity in the mediterranean region of South Africa. Hydrobiologia 719, 237–268 (2013).
James, N. et al. Effects of climate change on South African estuaries and associated fish species. Clim. Res. 57, 233–248 (2013).
Otto, F. E. L. et al. Anthropogenic influence on the drivers of the Western Cape drought 2015–2017. Environ. Res. Lett. 13, 124010 (2018).
Sydeman, W. J. et al. Climate change and wind intensification in coastal upwelling ecosystems. Science 345, 77–80 (2014).
Slingsby, J. A. et al. Intensifying postfire weather and biological invasion drive species loss in a Mediterranean-type biodiversity hotspot. Proc. Natl Acad. Sci. Usa. 114, 4697–4702 (2017).
Underwood, E. C., Viers, J. H., Klausmeyer, K. R., Cox, R. L. & Shaw, M. R. Threats and biodiversity in the mediterranean biome. Diversity Distrib. 15, 188–197 (2009).
Humphreys, A., Govaerts, R., Ficinski, S., Nic Lughadha, E. & Vorontsova, M. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evolution 3, 1–5 (2019).
Linder, H. P. The radiation of the Cape flora, southern Africa. Biol. Rev. 78, 597–638 (2003).
Slingsby, J. A., Ackerly, D. D., Latimer, A. M., Linder, H. P. & Pauw, A. The assembly and function of Cape plant communities in a changing world. in Fynbos: Ecology, evolution, and conservation of a megadiverse region. Eds: Allsopp, Nicky; Colville, Jonathan F.; Verboom, G. Anthony. 200–223 (Oxford University Press, 2014).
Cao, Q., Yu, G. & Qiao, Z. Application and recent progress of inland water monitoring using remote sensing techniques. Environ. Monit. Assess. 195, 125 (2023).
Mouw, C. B. et al. Aquatic color radiometry remote sensing of coastal and inland waters: Challenges and recommendations for future satellite missions. Remote Sens. Environ. 160, 15–30 (2015).
Palmer, S. C. J., Kutser, T. & Hunter, P. D. Remote sensing of inland waters: Challenges, progress and future directions. Remote Sens. Environ. 157, 1–8 (2015).
Botts, E. A. et al. Practical actions for applied systematic conservation planning. Conserv. Biol. 33, 1235–1246 (2019).
Cardoso, A. W. et al. BioSCape combines local knowledge and remote sensing technology for inclusive biodiversity science. Nature Reviews Biodiversity (in press).
Cardoso, A. W. et al. Increasing Data Access in Multi-Sensor Airborne Campaigns: Lessons from BioSCape in South Africa. in IGARSS 2024 - 2024 IEEE International Geoscience and Remote Sensing Symposium 2898–2901. https://doi.org/10.1109/IGARSS53475.2024.10641184 (IEEE, Athens, Greece, 2024).
ORNL DAAC. Biodiversity Survey of the Cape (BioSCape). Available at: https://daac.ornl.gov/cgi-bin/dataset_lister.pl?p=51 (2024).
Okujeni, A., Van Der Linden, S., Tits, L., Somers, B. & Hostert, P. Support vector regression and synthetically mixed training data for quantifying urban land cover. Remote Sens. Environ. 137, 184–197 (2013).
Frye, H. A. et al. Plant spectral diversity as a surrogate for species, functional and phylogenetic diversity across a hyper‐diverse biogeographic region. Glob. Ecol. Biogeogr. 30, 1403–1417 (2021).
Gamon, J. A. et al. Consideration of Scale in Remote Sensing of Biodiversity. in Remote Sensing of Plant Biodiversity (eds. Cavender-Bares, J., Gamon, J. A. & Townsend, P. A.) 425–447. https://doi.org/10.1007/978-3-030-33157-3_16 (Springer International Publishing, Cham, 2020).
Schweiger, A. K. et al. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2, 976–982 (2018).
Cawse-Nicholson, K., Damelin, S. B., Robin, A. & Sears, M. Determining the Intrinsic Dimension of a Hyperspectral Image Using Random Matrix Theory. IEEE Trans. Image Process. 22, 1301–1310 (2013).
Cawse-Nicholson, K., Hook, S. J., Miller, C. E. & Thompson, D. R. Intrinsic Dimensionality in Combined Visible to Thermal Infrared Imagery. IEEE J. Sel. Top. Appl. Earth Observations Remote Sens. 12, 4977–4984 (2019).
Cawse-Nicholson, K. et al. Surface Biology and Geology imaging spectrometer: A case study to optimize the mission design using intrinsic dimensionality. Remote Sens. Environ. 290, 113534 (2023).
Thompson, D. R., Boardman, J. W., Eastwood, M. L. & Green, R. O. A large airborne survey of Earth’s visible-infrared spectral dimensionality. Opt. Express 25, 9186 (2017).
Mokany, K., Ware, C., Woolley, S. N. C., Ferrier, S. & Fitzpatrick, M. C. A working guide to harnessing generalized dissimilarity modelling for biodiversity analysis and conservation assessment. Glob. Ecol. Biogeogr. 31, 802–821 (2022).
White, P. A., Frye, H. A., Slingsby, J. A., Silander, J. A. & Gelfand, A. E. Generative spatial generalized dissimilarity mixed modelling spGDMM: An enhanced approach to modelling beta diversity. Methods Ecol. Evol. 15, 214–226 (2024).
Luis, K., Cawse-Nicholson, K., Thompson, D. R., Smith, J. & Gierach, M. M. Estimating Phytoplankton Diversity with Intrinsic Dimensionality. 2022, GC33C-01 (2022).
Féret, J.-B. & Asner, G. P. Mapping tropical forest canopy diversity using high‐fidelity imaging spectroscopy. Ecol. Appl. 24, 1289–1296 (2014).
Rocchini, D. et al. The Spectral Species Concept in Living Color. JGR Biogeosciences 127, e2022JG007026 (2022).
Singh, A., Serbin, S. P., McNeil, B. E., Kingdon, C. C. & Townsend, P. A. Imaging spectroscopy algorithms for mapping canopy foliar chemical and morphological traits and their uncertainties. Ecol. Appl. 25, 2180–2197 (2015).
Wang, R. & Gamon, J. A. Remote sensing of terrestrial plant biodiversity. Remote Sens. Environ. 231, 111218 (2019).
Wang, Z. et al. Foliar functional traits from imaging spectroscopy across biomes in eastern North America. N. Phytologist 228, 494–511 (2020).
Serbin, S. P. & Townsend, P. A. Scaling Functional Traits from Leaves to Canopies. in Remote Sensing of Plant Biodiversity (eds. Cavender-Bares, J., Gamon, J. A. & Townsend, P. A.) 43–82. https://doi.org/10.1007/978-3-030-33157-3_3 (Springer International Publishing, Cham, 2020).
Asner, G. P. et al. Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 355, 385–389 (2017).
Meireles, J. E. et al. Leaf reflectance spectra capture the evolutionary history of seed plants. N. Phytologist 228, 485–493 (2020).
Cavender‐Bares, J. et al. Remotely detected aboveground plant function predicts belowground processes in two prairie diversity experiments. Ecol. Monogr. 92, e01488 (2022).
Dierssen, H. M. et al. Living up to the Hype of Hyperspectral Aquatic Remote Sensing: Science, Resources and Outlook. Front. Environ. Sci. 9, 649528 (2021).
Guild, L. S., Kudela, R. M., Hooker, S. B., Palacios, S. L. & Houskeeper, H. F. Airborne Radiometry for Calibration, Validation, and Research in Oceanic, Coastal, and Inland Waters. Front. Environ. Sci. 8, 585529 (2020).
Salama, M. S. & Stein, A. Error decomposition and estimation of inherent optical properties. Appl. Opt. 48, 4947 (2009).
Bell, T. W., Cavanaugh, K. C. & Siegel, D. A. Remote monitoring of giant kelp biomass and physiological condition: An evaluation of the potential for the Hyperspectral Infrared Imager (HyspIRI) mission. Remote Sens. Environ. 167, 218–228 (2015).
Hestir, E., Dronova, I., & University of California, Berkeley. Remote Sensing of Primary Producers in the Bay-Delta. SFEWS 20, (2023).
Hestir, E. L. et al. Measuring freshwater aquatic ecosystems: The need for a hyperspectral global mapping satellite mission. Remote Sens. Environ. 167, 181–195 (2015).
Bernard, S., Probyn, T. A. & Quirantes, A. Simulating the Optical Properties of Phytoplankton Cells Using a Two-Layered Spherical Geometry. Biogeosciences Discussions (2009).
Qi, J. et al. LESS: LargE-Scale remote sensing data and image simulation framework over heterogeneous 3D scenes. Remote Sens. Environ. 221, 695–706 (2019).
Lain, L. & Bernard, S. The Fundamental Contribution of Phytoplankton Spectral Scattering to Ocean Colour: Implications for Satellite Detection of Phytoplankton Community Structure. Appl. Sci. 8, 2681 (2018).
Masemola, C., Cho, M. A. & Ramoelo, A. Towards a semi-automated mapping of Australia native invasive alien Acacia trees using Sentinel-2 and radiative transfer models in South Africa. ISPRS J. Photogramm. Remote Sens. 166, 153–168 (2020).
Slingsby, J. A., Moncrieff, G. R., Rogers, A. J. & February, E. C. Altered ignition catchments threaten a hyperdiverse fire‐dependent ecosystem. Glob. Change Biol. 26, 616–628 (2020).
Kravitz, J., Matthews, M., Lain, L., Fawcett, S. & Bernard, S. Potential for High Fidelity Global Mapping of Common Inland Water Quality Products at High Spatial and Temporal Resolutions Based on a Synthetic Data and Machine Learning Approach. Front. Environ. Sci. 9, 587660 (2021).
Yao, W., Kelbe, D., Leeuwen, M., Romanczyk, P. & Aardt, J. Towards an Improved LAI Collection Protocol via Simulated and Field-Based PAR Sensing. Sensors 16, 1092 (2016).
Van Wilgen, B. W., Zengeya, T. A. & Richardson, D. M. A review of the impacts of biological invasions in South Africa. Biol. Invasions 24, 27–50 (2022).
Kraaij, T., Baard, J. A., Arndt, J., Vhengani, L. & van Wilgen, B. W. An assessment of climate, weather, and fuel factors influencing a large, destructive wildfire in the Knysna region, South Africa. fire ecol. 14, 4 (2018).
Kraaij, T. & van Wilgen, B. W. Drivers, ecology, and management of fire in fynbos. in Fynbos (eds. Allsopp, N., Colville, J. F. & Verboom, G. A.) 47–72. https://doi.org/10.1093/acprof:oso/9780199679584.003.0003 (Oxford University Press, 2014).
Le Maitre, D. C., Versfeld, D. B. & Chapman, R. A. Impact of invading alien plants on surface water resources in South Africa: a preliminary assessment. (2000).
Moncrieff, G. R., Slingsby, J. A. & Le Maitre, D. C. Propagating uncertainty from catchment experiments to estimates of streamflow reduction by invasive alien plants in southwestern South Africa. Hydrological Processes 35, (2021).
Rebelo, A. J. et al. The hydrological impacts of restoration: A modelling study of alien tree clearing in four mountain catchments in South Africa. J. Hydrol. 610, 127771 (2022).
Murray, B. R., Hose, G. C., Eamus, D. & Licari, D. Valuation of groundwater-dependent ecosystems: a functional methodology incorporating ecosystem services. Aust. J. Bot. 54, 221 (2006).
Barbier, E. B. et al. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193 (2011).
Buchanan, C. A Water Quality Binning Method to Infer Phytoplankton Community Structure and Function. Estuaries Coasts 43, 661–679 (2020).
Eger, A. M. et al. The value of ecosystem services in global marine kelp forests. Nat. Commun. 14, 1894 (2023).
Matthews, M. W. Eutrophication and cyanobacteria blooms in South African inland waters: 10 years of MERIS observations. Remote Sensing of Environment (2014).
Pitcher, G. C. et al. Devastating farmed abalone mortalities attributed to yessotoxin-producing dinoflagellates. Harmful Algae 81, 30–41 (2019).
Deiner, K., Fronhofer, E. A., Mächler, E., Walser, J.-C. & Altermatt, F. Environmental DNA reveals that rivers are conveyer belts of biodiversity information. Nat. Commun. 7, 12544 (2016).
Singh, S. et al. Metabarcoding of marine zooplankton in South Africa. Afr. J. Mar. Sci. 43, 147–159 (2021).
Browder, S. F., Johnson, D. H. & Ball, I. J. Assemblages of breeding birds as indicators of grassland condition. Ecol. Indic. 2, 257–270 (2002).
Depraetere, M. et al. Monitoring animal diversity using acoustic indices: Implementation in a temperate woodland. Ecol. Indic. 13, 46–54 (2012).
Siddig, A. A. H., Ellison, A. M., Ochs, A., Villar-Leeman, C. & Lau, M. K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 60, 223–230 (2016).
Sueur, J., Pavoine, S., Hamerlynck, O. & Duvail, S. Rapid Acoustic Survey for Biodiversity Appraisal. PLoS ONE 3, e4065 (2008).
Acknowledgements
We would like to acknowledge the invaluable contributions of the entire BioSCape team, including scientists, engineers, field technicians, data analysts, and support staff. The United States National Aeronautics and Space Administration (NASA) funded the U.S. components of the BioSCape campaign including airborne remote sensing instruments through NASA Grant #80NSSC21K0086 to A.M.W. and E.L.H. J.S. and G.M. were funded through National Research Foundation Grant #142438 and the South African Environmental Observation Network (SAEON). We further acknowledge the contributions of the South African National Research Foundation (NRF), which funded two affiliated South African-led research projects. The South African Environmental Observation Network (SAEON) provided in-kind support, access to research platforms and sites, and supported several capacity-building activities. J.S. further thanks UNESCO and the Government of The Netherlands for providing additional funding for capacity building and support for young South African researchers. The BioSCape project was also supported by NASA projects #NNX16AQ45G to A.M.W., #NNX16AQ34G to E.L.H., and #21-SMDSS21-0041 to PGB. JN was supported by the National Science Foundation Graduate Research Fellowship Program (NSF-GRFP) #2139297. This manuscript was improved by thoughtful feedback from two reviewers and the editor.
Author information
Authors and Affiliations
Contributions
A.W.C.: conceptualization; methodology; investigation; writing (original draft); writing (review and editing); visualization; project administration; and funding acquisition. E.L.H.: conceptualization; methodology; investigation; writing (reviewing and editing); visualization; resources; supervision; project administration; and funding acquisition. J.S.: conceptualization; methodology; investigation; writing (review and editing); project administration; and funding acquisition. C.J.F.: writing (review and editing). G.M.: conceptualization, investigation, writing (review and editing). A.S.: writing (review and editing); visualization. W.T.: writing (review and editing); conceptualization; methodology; resources; supervision; project administration; and funding acquisition. K.G.: conceptualization; methodology; resources; supervision; project administration; and funding acquisition. P.G.B.: methodology, investigation, writing (reviewing and editing). J.N.: investigation; writing (review and editing); visualization. A.M.W.: conceptualization; methodology; investigation; resources; writing (review and editing); visualization; supervision; project administration; and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cardoso, A.W., Hestir, E.L., Slingsby, J.A. et al. The biodiversity survey of the Cape (BioSCape), integrating remote sensing with biodiversity science. npj biodivers 4, 2 (2025). https://doi.org/10.1038/s44185-024-00071-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44185-024-00071-5