Abstract
Despite generating a great deal of interest in the form of review papers, progress in exploiting social dynamics for treatment strategies against bacterial infection has made limited progress since it was suggested twenty years ago. In contrast, anti-viral strategies based on social interactions are entering clinical trial stage. We explore possible reasons for this difference and highlight areas where the two fields of research may learn from one another.
Similar content being viewed by others
Introduction
Many microorganisms depend upon cooperative interactions. In bacteria, cooperation often involves the secretion of extracellular public goods, such as siderophore molecules to enable iron uptake1. In viruses, cooperation often involves the production of shared intracellular public goods, such as replicase enzymes for replicating the viral genome2. In both cases, cheat mutants emerge, which can gain a selfish evolutionary advantage by using these public goods, without producing them. The spread of such cheats can reduce the virulence of an infection, inspiring a conceptually new approach towards combatting bacterial and viral pathogens: could we use cheats to manipulate the social dynamics of clinical infections for therapeutic purposes?
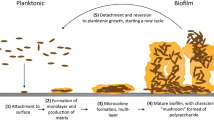
Fifteen years ago, Brown et al. applied the concept of the Trojan Horse to describe one way of exploiting cooperator-cheat dynamics in intervention strategies against bacterial infections3. This strategy relies on the exploitation of the ability of social cheats to invade a population of wildtype, cooperative bacteria. Cheats can be genetically engineered to carry medically beneficial alleles such as antibiotic sensitivity into an antibiotic-resistant population of cells causing an infection. It builds on the more basic idea of “treating with cheats”, which simply relies on the improvement of clinical outcomes by replacing more virulent wild-type (wt) cooperators with less virulent cheats (Fig. 1)4. The Trojan Horse idea has generated some excitement and enthusiasm for one major reason: it provides a possible alternative or complementary strategy to more conventional, drug-based strategies5,6,7,8,9.
A The invasive potential of social cheats can be exploited to drive medically beneficial alleles into a target population. However, the ability of this to be successful depends on a number of factors, some of which are listed. The good news is that evolutionary biology generates theoretical predictions about which conditions are likely to be more favourable. [Image modified from “The Procession of the Trojan Horse into Troy” by Giovanni Domenico Tiepolo, under creative Common License V4, creativecommons.org/licenses/by-nc-nd/4.0/]. B The process for implementing an intervention strategy based on the ability of bacterial cells or viral genomes to act as social cheats. The left-hand column illustrates the “treat with cheats” strategy: infected hosts are inoculated with a cheat strain which disrupts the ability of the infecting population to survive and/or reproduce; the resulting cheat-only population may have reduced virulence, or in the case of obligate cheating common in viruses, go extinct. The right-hand column illustrates use of the Trojan Horse strategy to reduce bacterial population resistance to antibiotics.
In this review, we explore the reasons why, despite the idea hardly losing momentum in terms of interest, researchers have made almost zero progress towards turning the Trojan Horse dream into Trojan Horse reality against bacterial infections. We contrast this with the widespread empirical success of cheat therapy in viruses. We discuss why the biology of viruses and bacteria have led to such contrasting empirical patterns, and suggest ways in which each field may learn from the other.
A short history of social evolution and bacterial infections
The first papers to emerge showing cooperator-cheat dynamics in bacteria10,11 were almost immediately followed by work exploring their potential for clinical applications. Andre & Godelle, presented one of the first and most influential examples of this, with a model that demonstrated the advantages of targeting social organisation within bacterial populations, rather than relying on killing populations cell-by-cell as antibiotics aim to do12. For example, bacteria rely on quorum sensing (QS) to coordinate density-dependent traits such as the release of virulence factors13. By disrupting quorum sensing, the virulence and/or ability of bacteria to proliferate and survive in the host environment may be significantly compromised. This strategy is known as quorum-quenching, and is one of the best studied examples of an intervention strategy which relies on an understanding of sociality in bacteria. For excellent reviews of virulence-targeting strategies, including quorum quenching, see Rasko and Sperandio (2010) and Martinez et al.14,15.
Treating with cheats – virulence and public goods
The most common form of cooperative behaviour in bacteria is contribution to a shared resource pool of excreted products, commonly referred to as “public goods”1. Examples of public goods include compounds involved in nutrient acquisition such as proteases and siderophores, bacteriocins involved in defence, and polymers involved in biofilm formation16. The signal molecules used by cells in quorum sensing are also a kind of public good10. Production of public goods fulfils the formal evolutionary definition of cooperation as they are costly to produce, beneficial to neighbouring cells and have been produced at least partly as a result of that benefit17. Compounds do not have to be excreted in order to fulfil the definition of a public good; enzymes degrade aminogylycoside antibiotics intracellularly, but this still contributes to detoxification of their environment, benefitting neighbouring cells18. Crucially, groups of cooperative individuals can be vulnerable to invasion by cheat strains that do not pay the cost of contributing to the public good pool but still benefit from the contribution of others11. This process has been well-documented in experimental studies but has also been shown to occur in infectious populations19,20. Cooperators can potentially evolve resistance to exploitation, perhaps by producing public goods that can only be utilised by relatives21 or by switching function to a private mechanism22. However, this is likely to be a relatively complex adaptation, requiring simultaneous mutations across multiple genes. Experimental work suggests that the most commonly favoured strategy is to become less cooperative to reduce the cost of exploitation, thus reducing the virulence of the infection (a strategy we might recognise as “if you can’t beat them, join them”)23.
There is broad overlap between compounds classed as public goods and virulence factors: products that contribute to host morbidity, which are typically secreted24. This means that disruption of cooperation through introduction of cheats can potentially reduce virulence; sometimes referred to as the “treat with cheats” strategy5. Potential efficacy of this strategy has been supported with experimental evidence. Biofilm formation through cooperative secretion of sticky polysaccharides presents a major challenge to treatments which rely on contact killing. Dieltjens et al.25 degraded biofilm structure of Salmonella culture, in vitro, by introduction of a competitive mutant that did not contribute to exo-polysaccaride production25. They went on to show that this had the effect of reducing antibiotic resistance within the population.
Two papers have demonstrated the potential of the “treat with cheats” strategy for bacteria in vivo, a crucial step towards exploiting its potential for medical benefit. Firstly, Harrison et al.26 infected waxmoth larvae by injection with Pseudomonas aeruginosa and reported reduced virulence when comparing wt with strains that were unable to produce the siderophore, pyoverdine26. Secondly, Rumbaugh et al.4 demonstrated the potential of the “treat with cheats” strategy against P. aeruginosa infections of murine hosts using QS mutants4. Mice infected with cheat strains had around 80% survival, compared to less than 40% when infected by wt. Crucially, this survival advantage was also seen in mice infected with mixed inoculum, where mortality rates were comparable with single strain cheat infections. Furthermore, systemic infection was only observed if wt cells were present. QS mutant, cheat strains, were restricted to the skin around the point of inoculation in single-strain infections but were harvested from the liver when co-inoculated with wild-type. The reasons for this difference are not known but it suggests that QS mutants are able to exploit the ability of wt cells to infiltrate host tissue: “treating with cheats” has the potential to be far more effective if there is nowhere for wt cells to hide.
Given the clarity of these results, it is perhaps surprising that in the intervening fifteen years, there are not more examples where researchers have attempted this, especially as advances in genetic modification have opened up new possibilities for optimising invasive potential27. It would be valuable to know, for example, if treating with cheats would work in a natural infection system, rather than lab infection systems chosen for experimental tractability. We need a better understanding of spatial organisation in host systems and how it influences cooperator-cheat dynamics28. Are some classes of public goods more suitable than others to this approach? Can the introduction of cheater strains to already-established infections improve survival, or does their ability to infiltrate wt infective populations rely on co-inoculation? The lack of research activity may be the result of publication bias – perhaps there are examples of failure to treat with cheats sitting in desk drawers, or it may be because these experiments are very difficult to perform. Another reason could be that there is a reluctance to invest in a strategy that ultimately involves inoculating a host with a potential pathogen. These are all understandable reasons but it is worth noting that progress is slow to non-existent.
Trojan Horse
In 2009, Brown et al. proposed an extension to the “treat with cheats” strategy, which exploits the invasive ability of social cheats to drive medically beneficial traits into an infective population. The authors used the analogy of the Trojan Horse to describe the idea (Fig. 1)3. To remind you, the Trojan Horse was the brainchild of wily Odysseus, Grecian warlord. After ten years of failure to breach the city gates of Troy, he presented the Trojans with a giant wooden horse as a “peace offering”, only to fill it with savage Greek warriors who attacked once inside the city walls. In our case: Troy = a infective population of cooperative wt bacterial cells; Horse = cheater strains capable of infiltration, which can be genetically engineered to transport Greeks = medically beneficial alleles that confer traits such as antibiotic sensitivity or quorum quenching. This strategy may be thought of as genetic engineering at the population scale: manipulation of population allele frequencies analogous to manipulation of an individual’s genotype by genetic engineering.
One of the key advantages of this strategy is its potential to combat antibiotic resistant populations. Could we engineer populations of resistant bacterial cells to become sensitive again to antibiotics which may otherwise be past their useful shelf-life? In this way, the Trojan Horse presents a possible means for escaping the Sisyphean task of maintaining a pipeline of new antibiotic drugs to combat bacterial infection; or at least offering an alternative strategy in less life-threatening cases to extend the shelf-life of existing antibiotics29. It can theoretically be applied to any species of bacteria and with growing interest in personalised medicine, can be adapted to exploit specific weaknesses in a particular infection. It’s a vivid example of how evolutionary biology can be applied to a global challenge facing humanity: instead of battling against evolutionary forces, we can harness evolution in bacteria for our advantage. For these reasons, it has generated a lot of interest and continues to be one of the topics most exciting and inspiring to students. The problem is that this interest has mainly manifested in the production of review papers and has resulted in minimal evidence to demonstrate its potential in the lab. If you’ll forgive us, one of the main motivations for writing this review is to ask – why is this a field composed almost entirely of reviews?
Empirical support for the Trojan Horse
There is a rich body of theory that has been tested in bacterial systems which predicts the likelihood of cheats successfully invading a population of cooperative cells – depending on cell density30, relative frequency of cheats to cooperators31, population viscosity11,32, the durability and diffusion distance of public good molecules33, and how costly the cooperative behaviour is to perform34,35. Harrison et al. demonstrate the context dependency of cooperator-cheat dynamics across a range of different model systems in the lab: cheats were able to invade in some systems but not in others, including those specifically designed to replicate conditions in the host36. All of these will vary within hosts, between points of infection, between hosts of the same species and across species. It will also vary between bacterial species, and genotypes within species. And while we can predict variation in potential efficacy to an extent, the challenge is defining important parameters in a living host, living in a natural population. In the absence of being able to identify the most likely scenario for success, the only other option may be trial and error.
A recent attempt to experimentally demonstrate the potential of the Trojan Horse strategy was conducted in vitro and in an ex vivo murine model37. In this study, the authors attempted to engineer a population of antibiotic resistant and highly virulent strain of P. aeruginosa into one that could be treated with antibiotics (Horse = QS mutant (lasR) and Greeks = antibiotic resistance). The experiments in liquid media showed some success – survival of treated cells was reduced by almost half following introduction of cheater strains, even in treatments where the antibiotic concentration was 4xMIC of the cheat. A set of experiments conducted in viscous media also showed reduction of resistance, although to a lesser extent. This is significant as spatial structuring is known from evolutionary theory to favour the maintenance of cooperation and bacterial cells infecting a host are likely to live in biofilm28. This is supported by Granato et al.38, who show that production of virulence factors significantly dropped in unstructured environments both in the presence and absence of the host, but remained unchanged in spatially structured environments38.
Mutlu et al. went on to conduct experiments in an ex vivo murine model and again, reduction of resistance was observed37. Despite the promising results of this study, however, the bacterial load following antibiotic treatment remained high with a two-fold reduction to 106 CFU/g. This is two orders of magnitude higher than the threshold usually considered to be necessary for successful healing by conventional drug-based therapies and remaining cells are more likely to be resistant39. The nature of the study system used by Multu et al. meant that there is no indication of the consequences of the cooperator-cheat dynamics for virulence - this would be an important consideration for inclusion by future studies. Furthermore, due to the context-dependency, the most appropriate systems for testing other Trojan Horse constructs would perhaps lend themselves to high degree of replication and minimal ethical concerns, such as in planta40.
What are the challenges and most promising strategies going forward?
Gurney et al. review the challenges involved in this field of research and highlight in particular the relatively low impact of evolution-based treatments on bacterial load, relative to antibiotics on bacterial load5. Another review by Waldetoft and Brown29 state “novel types of therapeutics. share the problem that, plainly put, they are not good enough for the treatment of severe infections”. However plausible, the evidence-base for understanding the potential of cheat-based therapies is limited, and existing studies report significant impact on survival in acute infection: Rumbaugh et al. reduced mortality in an acute infection model by over 50%4. The choice of species in these experiments, P. aeruginosa, is important here as it is notoriously difficult to treat with antibiotics. And the unfavourable comparison with drug-based treatments may change in the future, with the spread of antibiotic resistance.
Rumbaugh et al. were only able to achieve their result by co-inoculating QS wt (cooperator strains) with QS- (cheater strains); the same result was not observed when cheats were applied to an established infection of wt4. This has also been cited as a reason to question the potential efficacy of strategies that are dependent on the ability of cheats to invade an already-established infection. Again, this conclusion is based on a single study which is not consistent across infection systems: Mutlu et al. were able to drive antibiotic sensitivity into an already established resistant population of bacteria in an ex vivo murine infection model37. While a note of caution and scepticism is appropriate, our argument here is that there is a danger of making too many assumptions from too little data collected in too few infection systems. See Box 1 for some discussion of outstanding issues in relation to clinical applications.
Successful cheat therapy in viruses
In contrast, there are many successful examples of cheat therapy being applied against viral infections (Table 1). The field has developed very differently in virology, having been driven primarily by experimental and clinical virologists, rather than evolutionary biologists. In some cases, virologists have independently defined terms that are also used in evolutionary theory, reflecting both substantial conceptual overlap as well as a historical lack of cross-talk between these fields2. Here, we provide a brief summary of how social evolution has been used in clinical applications within virology, highlighting some success stories and future opportunities.
Viruses also cheat
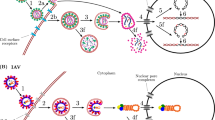
Like bacteria, viruses also depend upon public goods, in this case typically shared within coinfected host cells. Two common examples are replicase enzymes, which replicate the viral genome, and capsid proteins, which construct the virion required to transport viral genomes to new host cells2. Both types of gene product can be used by viral genomes that did not contribute to their production. Cheats therefore arise relatively easily through deletions in genes for public goods (Fig. 2). In virology, these large-deletion mutants are referred to as ‘defective interfering genomes’41 and they are defined by almost exactly the same properties that are used to define cheats in social evolution42.
Two common examples include replicase enzymes for replicating the viral genome (here in yellow) or capsid protein subunits required for building the viral capsid (here in blue). These gene products can be exploited by non-producing mutants, called cheats, which benefit from them without contributing. A Cheat mutants often arise spontaneously, when error-prone replication results in shorter viral genomes which lack the regions that encode shared gene products. B In coinfection, these cheat mutants exploit gene products encoded by full-length, cooperative viruses. Over the course of a cellular infection, cheats can out-compete full-length viruses by up to 10,000:1, even driving extinction of the viral population. Image produced using Biorender®.
“Defective interfering genome” cheats are common, emerging in laboratory infections of almost all known viruses43. They have been studied for a very long time – the first attempts to grow viruses in cell culture were stymied by the accumulation of these cheats44 – and their therapeutic potential was quickly recognised41. While of the clinical use of defective interfering genomes is an example of cheat therapy, the term ‘cheat’ is rarely used. Instead, these therapeutic cheats are typically referred to as ‘Therapeutic Interfering Particles’ or TIPs2.
Cheat therapy works in viruses
In contrast with the bacterial field, there are many examples of viral cheat therapy working successfully in vivo (Table 1). Cheat therapy has been successful across diverse viral taxa, from RNA retroviruses such as HIV to large dsDNA viruses such as herpesvirus, as well as across different types of animal host, from insect vectors to non-human primates45,46,47. Cheat therapy is now starting to reach the clinic: the first clinical trials have recruited human volunteers; and there is substantial investment from government research agencies and commercial biotechnology companies.
A viral Trojan Horse?
While TIPs are powerful examples of the ‘cheat with treats’ strategy, to date, there have been relatively few examples of studies which adopt a ‘Trojan Horse’ strategy in viruses. One notable exception comes from therapeutic strategies developed against herpes viruses. Walter & Verdin designed a mutant version of human cytomegalovirus with a CRISPR-based gene drive48. Upon coinfecting with wild-type virus, the gene drive cuts the wild-type viral genome, which is then repaired by homologous recombination in a way that converts wild-type viruses into mutant gene drive viruses. This approach was successful at replacing essential herpes virus genes with new versions that reduced viral fitness. More recent work has extended this approach to herpes simplex virus 1 and showed that such a gene drive can spread in vivo in an established infection47.
The social nature of many key viral traits suggests other Trojan Horse strategies that could offer benefits beyond existing viral cheat therapies. For example, in coinfection, many viruses build mixed virions that contain proteins encoded by different genomes. A cheat virus that also encoded a detrimental phenotype could provide a two-fold therapeutic benefit by sensitising wild-type genomes within mixed virions to drugs or the immune system49,50.
Why might cheat therapy be more effective in viruses?
In principle, many of the barriers to cheat therapy in bacteria should also apply to viruses. Viral cheats can only spread when there are high levels of coinfection and low relatedness, and it is not clear whether these conditions are met in natural infections. Furthermore, we know that natural viral infections exhibit substantial spatial structure, fluctuating population sizes, and repeated population bottlenecks both within and between hosts51,52,53. All of these factors should disfavour cheat spread, and should also vary significantly among different viruses and hosts. Given these shared barriers, why are there so many examples of cheat therapy working in viruses, and so few in bacteria?
A number of key factors may allow viral cheats to be more effective therapeutics than bacterial cheats; for example:
-
1.
Viral cheats can achieve extraordinarily large fitness advantages. For example, in poliovirus, a coinfected cell can produce 10,000 times as many cheat virions as cooperator virions54. In contrast, for bacteria, cheat advantages are typically at least three orders of magnitude lower, even in in vitro conditions designed to maximise chance of mutant invasion34. Viral cheats may simply gain such large advantages that they can spread even when conditions are otherwise unfavourable.
-
2.
Secondly, viral cheats often stimulate host immune responses. In many of the examples in Table 1, viral cheats trigger a strong innate immune response, contributing to further suppression of the viral infection. The effect on host immunity is supported by lab studies showing that cheat genomes trigger disproportionately potent innate immune responses55. Thus, even in cases when their direct effect on cooperators may be weak, cheats may still cause a strong indirect effect that suppresses cooperators.
-
3.
Viral cheats often exploit essential functions, such as genome replication, whereas bacterial cheats often exploit non-essential functions, such as siderophore production. As a result, successful cheat invasion in bacteria may result in a less virulent, but still extant, population of pathogens, whereas in viruses, cheat invasion may result in the extinction of the pathogen population altogether.
More broadly, this growing literature could provide fertile ground to investigate the factors which enable successful cheat invasion. In particular, the viral literature contains diverse examples of successful cheat therapy, including in hosts that vary in spatial structure, across infections that vary on the chronic/acute axis, and where different types of viral social trait are exploited. Understanding these factors could allow us to identify promising therapeutic avenues going forward, in viruses, bacteria, and potentially other pathogenic organisms.
Pragmatic reasons may also have contributed to the comparatively enthusiastic adoption of cheat therapy in viruses. Viral cheats frequently emerge spontaneously when viruses are grown in the laboratory, which immediately allows high-throughput approaches for screening candidate cheat viruses56,57. Similarly, viral cheats have been studied since the earliest days of virology, across almost every type of virus that is grown in the laboratory. As a result, there is a large existing body of knowledge to draw upon for rational design of synthetic viral cheats, across a wide range of viruses43. In contrast, empirical work on bacterial cheating is a relatively younger field, and is confined to a smaller number of model systems.
What can viral cheat therapy learn from sociomicrobiology?
The widespread efficacy of cheat therapy in viruses has shifted the debate. While for bacterial cheat therapy, the question is, “will it work?”, for viruses, the relevant question is now, “is it safe?”. To address safety concerns, we need to predict the future consequences of manipulating social interactions, both within individual patients and among populations, and including in scenarios where data may be scarce. Making predictions of this kind is precisely the goal of evolutionary theory. However, in contrast to the bacterial field, therapeutic applications of viral cheating have only rarely been directly integrated with evolutionary theory58.
Specific safety concerns include those that depend upon within-host processes, such as whether cooperators will evolve resistance to cheats59,60, or whether cheats can prolong viral infections in a way that increases transmission opportunities61. Other concerns are about between-host processes, such as whether viral cheats can spread from host to host62. The answers to these questions are essential for regulation and for the design of clinical trials. For example, a therapy that can spread from person to person, such as the oral poliovirus vaccine, has different risks, benefits, and consent considerations than therapeutics that doesn’t spread, such as more recent mRNA vaccines.
In some cases, detailed mathematical models are available for specific therapeutic viral cheats, that can help to answer these questions62,63,64,65. These models have identified mechanistic design requirements of therapeutic cheats, but a greater integration with the broader field of coevolution theory could help resolve contrasting claims over the consequences of cheat/cooperator coevolution. Moving forward, we need both detailed modelling approaches for viruses in which therapeutic cheats are newly available, as well as broader modelling approaches that can use the principles of cheat/cooperator coevolution to predict epidemiological and evolutionary consequences across different types of virus, especially if integrated with the increasing body of data on naturally occurring viral cheats2,66,67,68. Such theoretical approaches have been used to great success in the field of gene drives, for assessing risk, efficacy, and developing new types of therapeutic strategy. We argue that a similar degree of quantitative integration is the crucial next step for viral cheat therapy.
A Trojan future?
Our review highlights the contrasting state of research into cheat therapy for bacterial and viral infections. Virologists have been highly successful at treating infections with cheats in vivo, and are developing promising avenues for Trojan Horse approaches. In contrast, there are relatively few published examples of cheat therapy being applied against bacterial infection. Greater integration between these fields would be mutually beneficial; virology research may act as a source of inspiration for bacterial cheat therapy, forging ahead in developing clinical applications. At the same time, viral cheat therapy stands to benefit from making greater use of evolutionary theory, especially for assessing crucial ethical and safety concerns. In short, evolutionary biology provides a powerful conceptual framework for developing novel antimicrobial strategies in bacteria and viruses. But without better integration between theory and data, it can go no further than that.
Data availability
No datasets were generated or analysed during the current study.
References
West, S. A., Griffin, A. S., Gardner, A. & Diggle, S. P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4, 597–607 (2006).
Leeks, A., West, S. & Ghoul, M. The evolution of cheating in viruses. Nat. Commun. 12, 2916 (2021).
Brown, S. P., West, S. A., Diggle, S. P. & Griffin, A. S. Social evolution in micro-organisms and a Trojan horse approach to medical intervention strategies. Philos. Trans. R. Soc. B 364, 3157–3168 (2009).
Rumbaugh, K. P. et al. Quorum sensing and the social evolution of bacterial virulence. Curr. Biol. 19, 341–345 (2009).
Gurney, J., Simonet, C., Waldetoft, K. & Brown, S. Challenges and opportunities for cheat therapy in the control of bacterial infections. Nat. Prod. Rep. 39, 325–334 (2022).
Lissens, M., Joos, M., Lories, B. & Steenackers, H. Evolution-proof inhibitors of public good cooperation: a screening strategy inspired by social evolution theory. FEMS Microbiol. Rev. 46, fuac024 (2022).
Rezzoagli, C., Granato, E. & Kümmerli, R. Harnessing bacterial interactions to manage infections: a review on the opportunistic pathogen Pseudomonas aeruginosa as a case example. J. Med. Microbiol. 69, 147–161 (2020).
Andersen, S., Shapiro, B., Vandenbroucke-Grauls, C. & de Vos, M. Microbial evolutionary medicine: from theory to clinical practice. Lancet Infect. Dis. 19, e273–e283 (2019).
García-Contreras, R. & Loarca, D. The bright side of social cheaters: potential beneficial roles of ‘social cheaters’ in microbial communities. FEMS Microbiol. Ecol. 97, fiaa214 (2021).
Diggle, S. P., Griffin, A. S., Campbell, G. S. & West, S. A. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–414 (2007).
Griffin, A., West, S. & Buckling, A. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027 (2004).
André, J. & Godelle, B. Multicellular organization in bacteria as a target for drug therapy. Ecol. Lett. 8, 800–810 (2005).
Darch, S. E., West, S. A., Winzer, K. & Diggle, S. P. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc. Natl. Acad. Sci. USA 109, 8259–8263 (2012).
Rasko, D. A. & Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 9, 117–128 (2010).
Martinez, O. F., Cardoso, M. H., Ribeiro, S. M. & Franco, O. L. Recent advances in anti-virulence therapeutic strategies. Front. Cell. Infect. Microbiol. 9, 34 (2019).
West, S. A., Diggle, S. P., Buckling, A., Gardner, A. & Griffin, A. S. The social lives of microbes. Annu. Rev. Ecol. Evol. Syst. 38, 53–77 (2007).
West, S. A., Griffin, A. S. & Gardner, A. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432 (2007).
Sorg, R. A. et al. Collective resistance in microbial communities by intracellular antibiotic deactivation. PLoS Biol 14, e2000631 (2016).
Köhler, T., Buckling, A. & Van Delden, C. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc. Natl. Acad. Sci. USA 106, 6339–6344 (2009).
Andersen, S. B., Marvig, R. L., Molin, S., Johansen, H. K. & Griffin, A. S. Long-term social dynamics drive loss of function in pathogenic bacteria. Proc. Natl. Acad. Sci. USA 112, 10756–10761 (2015).
Wall, D. Kin recognition in bacteria. Annu. Rev. Microbiol. 70, 143–160 (2016).
Andersen, S. B. et al. Privatisation rescues function following loss of cooperation. eLife 7, e38594 (2018).
Kümmerli, R. et al. Co-evolutionary dynamics between public good producers and cheats in the bacterium Pseudomonas aeruginosa. J. Evol. Biol 28, 2264–2274 (2015).
West, S. & Buckling, A. Cooperation, virulence and siderophore production in bacterial parasites. Proc. R. Soc. B 270, 37–44 (2003).
Dieltjens, L. et al. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 11, 1–10 (2020).
Harrison, F., Browning, L. E., Vos, M. & Buckling, A. Cooperation and virulence in acute Pseudomonas aeruginosa infections. BMC Biol 4, 21 (2006).
González, J. et al. Loss of a pyoverdine secondary receptor in Pseudomonas aeruginosa results in a fitter strain suitable for population invasion. ISME J. 15, 1330–1343 (2021).
Rezzoagli, C., Granato, E. T. & Kümmerli, R. In-vivo microscopy reveals the impact of Pseudomonas aeruginosa social interactions on host colonization. ISME J. 13, 2403–2414 (2019).
Waldetoft, K. W. & Brown, S. P. Alternative therapeutics for self-limiting infections – an indirect approach to the antibiotic resistance challenge. PLoS Biol. 15, e2001356 (2017).
Ross-Gillespie, A., Gardner, A., Buckling, A., West, S. A. & Griffin, A. S. Density dependence and cooperation: theory and a test with bacteria. Evolution 63, 2315–2325 (2009).
Ross-Gillespie, A., Gardner, A., West, S. A. & Griffin, A. S. Frequency dependence and cooperation: theory and a test with bacteria. Am. Nat. 170, 331–342 (2007).
Kümmerli, R., Griffin, A. S., West, S. A., Buckling, A. & Harrison, F. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. R. Soc. B 276, 3531–3538 (2009).
Kümmerli, R. & Brown, S. P. Molecular and regulatory properties of a public good shape the evolution of cooperation. Proc. Natl. Acad. Sci. USA 107, 18921–18926 (2010).
Jiricny, N. et al. Fitness correlates with the extent of cheating in a bacterium. J. Evol. Biol. 23, 738–747 (2010).
Ghoul, M. et al. Pyoverdin cheats fail to invade bacterial populations in stationary phase. J. Evol. Biol. 29, 1728–1736 (2016).
Harrison, F., McNally, A., da Silva, A., Heeb, S. & Diggle, S. Optimised chronic infection models demonstrate that siderophore ‘cheating’ in Pseudomonas aeruginosa is context specific. ISME J. 11, 2492–2509 (2017).
Mutlu, A., Vanderpool, E. J., Rumbaugh, K. P., Diggle, S. P. & Griffin, A. S. Exploiting cooperative pathogen behaviour for enhanced antibiotic potency: a Trojan horse approach. Microbiology 170, 1–9 (2024).
Granato, E. T., Ziegenhain, C., Marvig, R. L. & Kümmerli, R. Low spatial structure and selection against secreted virulence factors attenuates pathogenicity in Pseudomonas aeruginosa. Nat. Commun. 12, 2907–2918 (2018).
Edwards, R. & Harding, K. G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 17, 91–99 (2004).
Friesen, M. L. Social evolution and cheating in plant pathogens. Annu. Rev. Phytopathol. 58, 55–75 (2020).
Huang, A. S. & Baltimore, D. Defective viral particles and viral disease processes. Nature 226, 325–337 (1970).
Ghoul, M., Griffin, A. S. & West, S. A. Toward an evolutionary definition of cheating. Evolution 68, 318–331 (2014).
Vignuzzi, M. & López, C. B. Defective viral genomes are key drivers of the virus–host interaction. Nat. Microbiol. 1, 1–9 (2019).
von Magnus, P. Studies on interference in experimental influenza. Almqvist Wiksell 1, 120 (1947).
Pitchai, F. N. N. et al. Engineered deletions of HIV replicate conditionally to reduce disease in nonhuman primates. Science 385, eadn5866 (2024).
Rezelj, V. V. et al. Defective viral genomes as therapeutic interfering particles against flavivirus infection in mammalian and mosquito hosts. Nat. Commun. 12, 2290 (2021).
Walter, M. et al. Viral gene drive spread during herpes simplex virus 1 infection in mice. Nat. Commun. 15, 8161 (2024).
Walter, M. & Verdin, E. Viral gene drive in herpesviruses. Nat. Commun. 11, 4884 (2020).
Tanner, E. J. et al. Dominant drug targets suppress the emergence of antiviral resistance. eLife 3, e03830 (2014).
Tanner, E. J., Kirkegaard, K. A. & Weinberger, L. S. Exploiting genetic interference for antiviral therapy. PLoS Genet. 12, e1005986 (2016).
Amato, K. A. et al. Influenza A virus undergoes compartmentalized replication in vivo dominated by stochastic bottlenecks. Nat. Commun. 13, 3416 (2022).
Gutiérrez, S. et al. The multiplicity of cellular infection changes depending on the route of cell infection in a plant virus. J. Virol. 89, 9665–9675 (2015).
Zwart, M. P. & Elena, S. F. Matters of size: genetic bottlenecks in virus infection and their potential impact on evolution. Annu. Rev. Virol. 2, 161–179 (2015).
Shirogane, Y. et al. Experimental and mathematical insights on the interactions between poliovirus and a defective interfering genome. PLoS Pathog. 17, e1009277 (2021).
Manzoni, T. B. & López, C. B. Defective (interfering) viral genomes re-explored: impact on antiviral immunity and virus persistence. Future Virol 13, 1–9 (2018).
Levi, L. I. et al. Defective viral genomes from chikungunya virus are broad-spectrum antivirals and prevent virus dissemination in mosquitoes. PLoS Pathog. 17, e1009110 (2021).
Kupke, S. Y., Riedel, D., Frensing, T., Zmora, P. & Reichl, U. A novel type of influenza A virus-derived defective interfering particle with nucleotide substitutions in its genome. J. Virol. 93, https://doi.org/10.1128/jvi.01786-18 (2019).
Leeks, A. et al. Open questions in the social lives of viruses. J. Evol. Biol. 36, 1551–1567 (2023).
DePolo, N., Giachetti & Holland, J. Continuing coevolution of virus and defective interfering particles and of viral genome sequences during undiluted passages: virus mutants exhibiting nearly complete resistance to formerly dominant defective interfering particles. J. Virol. 61, 454–464 (1987).
Horiuchi, K. Co-evolution of a filamentous bacteriophage and its defective interfering particles. J. Mol. Biol. 169, 389–407 (1983).
Poirier, E. Z. et al. Dicer-2-dependent generation of viral DNA from defective genomes of RNA viruses modulates antiviral immunity in insects. Cell Host Microbe 23, 353–365.e8 (2018).
Metzger, V. T., Lloyd-Smith, J. O. & Weinberger, L. S. Autonomous targeting of infectious superspreaders using engineered transmissible therapies. PLoS Comput. Biol. 7, e1002015 (2011).
Ke, R. & Lloyd-Smith, J. O. Evolutionary analysis of human immunodeficiency virus type 1 therapies based on conditionally replicating vectors. PLoS Comput. Biol. 8, e1002744 (2012).
Rüdiger, D., Pelz, L., Hein, M. D., Kupke, S. Y. & Reichl, U. Multiscale model of defective interfering particle replication for influenza A virus infection in animal cell culture. PLoS Comput. Biol. 17, e1009357 (2021).
Rouzine, I. M. & Weinberger, L. S. Design requirements for interfering particles to maintain coadaptive stability with HIV-1. J. Virol. 87, 2081–2093 (2013).
Felt, S. A. et al. Detection of respiratory syncytial virus defective genomes in nasal secretions is associated with distinct clinical outcomes. Nat. Microbiol. 6, 1–10 (2021).
Martin, M. A., Berg, N. & Koelle, K. Influenza A genomic diversity during human infections underscores the strength of genetic drift and the existence of tight transmission bottlenecks. Virus Evol. 10, veae042 (2024).
Vasilijevic, J. et al. Reduced accumulation of defective viral genomes contributes to severe outcome in influenza virus infected patients. PLoS Pathog. 13, e1006650 (2017).
Welch, S. R. et al. Defective interfering viral particle treatment reduces clinical signs and protects hamsters from lethal Nipah virus disease. mBio 13, e03294-21 (2022).
Chaturvedi, S. et al. Identification of a therapeutic interfering particle – a single-administration SARS-CoV-2 antiviral intervention with a high barrier to resistance. Cell 0, 1–10 (2021).
Noble, S. & Dimmock, N. J. Defective interfering type A equine influenza virus (H3N8) protects mice from morbidity and mortality caused by homologous and heterologous subtypes of influenza A virus. J. Gen. Virol. 75, 3485–3491 (1994).
Acknowledgements
The authors thank Sam Brown and two anonymous reviewers for comments on the manuscript. A.S.G. acknowledges support from ERC Consolidator grant number 647586; A.L. acknowledges support from the James S McDonnell Foundation.
Author information
Authors and Affiliations
Contributions
A.S.G. and A.L. wrote the manuscript text, A.S.G. prepared Figure 1 and A.L. prepared Figure 2. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Griffin, A.S., Leeks, A. Exploiting social traits for clinical applications in bacteria and viruses. npj Antimicrob Resist 3, 20 (2025). https://doi.org/10.1038/s44259-025-00091-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44259-025-00091-6