Abstract
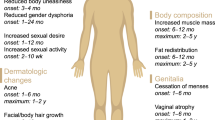
Editor's Note: Has the FDA discriminated against female sexual dysfunction by not endorsing Procter & Gamble's (P&G) Intrinsa? Has the pendulum swung so far to the right that drugs for sexual medicine receive undue scrutiny? Why is testosterone replacement therapy available for men, but not for women? How should the pharmaceutical industry proceed with future studies? How should clinicians guide their patients? In this third and final perspective regarding Intrinsa, Sheryl Kingsberg, PhD, an expert on female sexual dysfunction and one of the lead investigators of the Intrinsa clinical trials, addresses these questions. This is a highly charged issue that appears to be on the agenda for the foreseeable future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kingsberg, S. The testosterone patch for women. Int J Impot Res 17, 465–466 (2005). https://doi.org/10.1038/sj.ijir.3901375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901375