Journal Information
Journal ID (publisher-id): BM
Journal ID (nlm-ta): Biochem Med (Zagreb)
Title: Biochemia Medica
Abbreviated Title: Biochem. Med. (Zagreb)
ISSN (print): 1330-0962
ISSN (electronic): 1846-7482
Publisher: Croatian Society of Medical Biochemistry and Laboratory Medicine
Article Information
Copyright statement: ©Croatian Society of Medical Biochemistry and Laboratory Medicine.
Copyright: 2025, Croatian Society of Medical Biochemistry
License (open-access):
This is an Open Access article distributed under the terms of the Creative Commons Attribution (http://creativecommons.org/licenses/by/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Date received: 06 May 2024
Date accepted: 09 April 2025
Publication date: 15 June 2025
Publication date: 15 June 2025
Volume: 35
Issue: 2
Electronic Location Identifier: 020706
Publisher ID: bm-35-2-020706
DOI: 10.11613/BM.2025.020706
National recommendations of the Working group for laboratory diagnostics of autoimmune diseases of the Croatian Society of Medical Biochemistry and Laboratory Medicine: Assessment of antineutrophil cytoplasmic antibodies (ANCA)
Nada Tomić Sremec[1]
Vedrana Drvar[6]
Katarina Gugo[7]
[1] Department of Laboratory Diagnostics, University Hospital Centre Zagreb, Zagreb, Croatia
[2] University of Zagreb, Faculty of Pharmacy and Biochemistry, Zagreb, Croatia
[3] Department of Clinical Chemistry, Sestre Milosrdnice University Hospital Center, Zagreb, Croatia
[4] Catholic University of Croatia, Zagreb, Croatia
[5] Clinical Department of Laboratory Diagnostics, Dubrava University Hospital, Zagreb, Croatia
[6] Clinical Department of Laboratory Diagnostics, Clinical Hospital Center Rijeka, Rijeka, Croatia
[7] Medical Laboratory Diagnostic Division, University Hospital of Split, Split, Croatia
Author notes:
[*] Corresponding author: akozmar@kbc-zagreb.hr
Author contributions
A Kozmar: Conceptualization, Investigation, Writing - original draft. A Tešija Kuna: Conceptualization, Investigation, Writing - original draft. N Tomić Sremec: Investigation, Writing – review&editing. L Đerek: Investigation, Writing - review&editing. V Drvar: Investigation, Writing - review&editing. K Gugo: Investigation, Writing - review&editing.
• National recommendations for antineutrophil cytoplasmic antibodies (ANCA) testing based on the International consensuses on ANCA testing and reporting
• Recommendations include indications for ANCA testing, preanalytical, analytical and postanalytical issues, including rational algorithm and quality control assurance
• These recommendations aim to harmonize ANCA testing to enable their laboratory use and better understanding of their diagnostic value
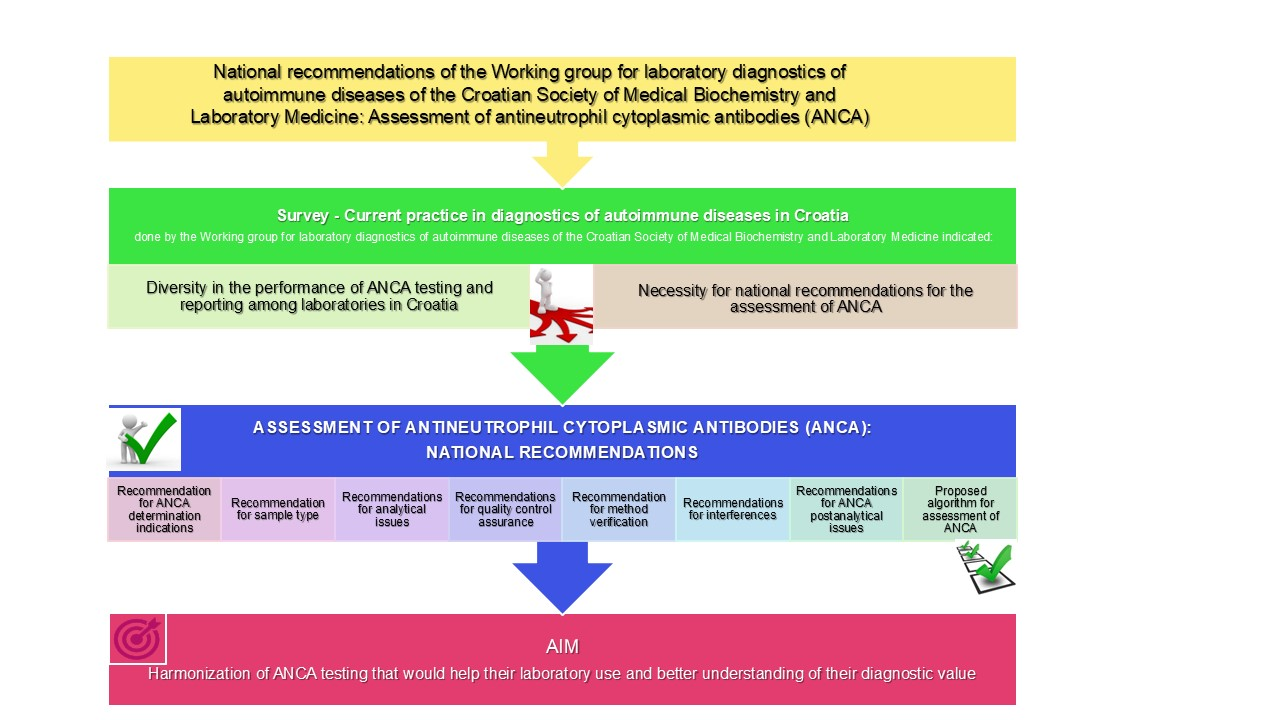
The family of antineutrophil cytoplasmic antibodies (ANCA) includes autoantibodies targeting proteins within the primary granules of neutrophils and lysosomes of monocytes. So far, proteinase 3 (PR3) and myeloperoxidase (MPO) are considered clinically relevant ANCA specificities. National recommendations for the assessment of ANCA are the outcome of the survey done by the Working group (WG) for laboratory diagnostics of autoimmune diseases of the Croatian Society of Medical Biochemistry and Laboratory Medicine (CSMBLM), where the diversity in the performance of ANCA testing and reporting among the laboratories in Croatia was observed. This document contains recommendations concerning the indications for ANCA testing, preanalytical, analytical and postanalytical issues, including rational algorithm and quality control assurance. The recommendations are based on the International consensus on ANCA testing and reporting as well as other relevant literature in order to help to harmonize ANCA testing. The aim of these recommendations is to improve and harmonize ANCA testing among laboratories in Croatia.
Keywords: antineutrophil cytoplasmic antibodies; autoimmunity; harmonization; recommendations