Abstract
Because of their uniform and regular channels, adjustable pore size, large surface area, controllable wall composition, high hydrothermal stability, ease of functional modification, and good accessibility of larger reactant molecules, mesoporous siliceous SBA-15 is of excellent catalyst carrier that is highly versatile and has been used extensively to prepare a variety of supported catalysts with ideal catalytic properties. In this study, we report the synthesis, characterization, and catalytic application of Cu-Ag/ SBA-15 nanoalloy catalysts towards the control of microorganisms in drinking water has been reported. The Cu-Ag/SBA-15 nanoalloy catalysts with different molar mass ratio of copper to silver (Cu:Ag = 1: 0, 0.75: 0.25, 0.5: 0.5, 0.25: 0.75, 0: 1) keeping 1weight % total loading of copper and silver metals on SBA-15 support have been prepared by incipient wetness impregnation method and characterized by various characterization techniques like, low angle XRD, wide angle XRD, N2-physcisorption and scanning electron microscopy techniques. The anti-bacterial activity of the catalysts was measured qualitatively by testing the presence of coliforms in water after contacting with the catalyst at room temperature. These nanoalloy catalysts found to be effective in controlling the microorganisms in drinking water. Among the series of the catalysts prepared, 0.25Cu-0.75Ag /SBA-15 catalyst showed superior catalytic activity. The high catalytic performance of the catalyst is due to its high surface area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Worldwide, there is now a severe crisis due to the growing need for clean water and the depletion of available supplies brought on by the growth of industries, population growth, and protracted droughts. It leads to a need for the creation of novel, workable, and financially appealing technologies that permit sensible water use. Millions of people die each year from illnesses brought on by contaminated water, with over 4 billion people thought to have extremely restricted access to clean water [1]. It is reasonable to anticipate that such figures will rise in the future in line with the rising levels of environmental contamination brought on by the deposition of dangerous compounds into the natural water cycle [2]. The development of low-cost, high-performing water and wastewater treatment technologies is necessary due to the low quality of natural water and clean water shortage [3, 4].
When used as standalone or combined procedures, traditional water treatment techniques (such as filtration, activated carbon adsorption, flocculation/sedimentation, coagulation/flocculation/ sedimentation, and chlorination) are unable to completely remove microorganisms and hazardous inorganic and organic compounds from drinking water sources. As a result, new methods of treating wastewater and water, such as membrane technology, UV disinfection, and "Advanced Oxidation Processes," are being developed [5, 6]. Nevertheless, those techniques do not result in the complete neutralization or elimination of contaminants—rather, they just allow for the concentration or transfer of impurities to another phase. Although chlorination is typically used in disinfection processes, it can also produce byproducts that are carcinogenic and mutagenic [6]. Reverse osmosis, ultrafiltration, nanofiltration, and microfiltration are examples of chemical and membrane processes that have significant operating costs and have the potential to produce hazardous secondary pollutants that enter ecosystems. Membrane technologies are severely constrained, even though they have emerged as a viable substitute for traditional treatment techniques [7]. First off, those procedures only guarantee the physical separation of organic and inorganic components, as well as microbes. Because of this, the concentrated stream—roughly 10% of the treated water volume—contains pathogenic microorganisms that are active and can seriously harm the feed stream or deposition site [7].
For millennia, people have been aware of metallic silver's antimicrobial properties [8]. The dissolution of silver ions from the bulk silver's surface is the source of this advantageous characteristic. Unlike when using silver ions, bulk silver has a stable and long-lasting biocidal impact. However, due to its high cost and slow ion release rate, bulk silver is challenging to employ in household or industrial settings. A new method for applying silver antibacterial agents has emerged recently with the use of silver nanoparticles due to their large surface to volume ratio. Numerous studies have been conducted to determine the bactericidal potential of nanoparticles and their potential uses in the paint, textile, health, and plastics industries [9,10,11,12,13,14]. However, in view of green chemistry requirements, it is necessary to use reusable heterogeneous catalysts in the disinfection water. It has been reported that the Ag based catalysts have been effectively controls the microorganisms in drinking water [15,16,17,18]. Mesoporous materials have attracted a lot of attention due to their potential use in absorption, separation, catalysis, and other processes. SBA-15 is among the best catalytic supports in the mesoporous material family due to its large and constant pore size, high porosity, and large surface area. These features provide a dependable and well-isolated environment for the creation of nanoparticles. [19,20,21] In the present study, we describe the preparation of Cu-Ag/SBA-15 nanoalloy catalysts and its catalytic activity in control of microorganisms in drinking water.
2 Experimental
2.1 Preparation of mesoporous SBA-15
Mesoporous silica SBA-15 was prepared accordance with previous methods. [19,20,21,22,23,24,25] In general, SBA-15 was prepared by hydrothermal method in acidic medium using P123 polymer as structure directing agent and TEOS as silica source. A solution of EO20PO70EO20: 2 M HCl: TEOS: H2O = 2: 60: 4.25: 15 (mass ratio) was prepared, stirred for 12 h at 40 °C and then hydrothermally treated at 100 °C under static conditions for 12 h, subsequently filtered, dried at 100 °C and calcined at 550 °C for 8 h, the yielded white powder is mesoporous silica SBA-15.
2.2 Preparation of Cu-Ag/SBA-15 catalysts
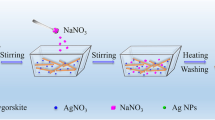
The Cu-Ag/SBA-15 catalysts have been prepared by incipient wetness impregnation method by taking the requisite amount of homogeneous mixed salt solutions of Cu and Ag with high dilution with the composition of copper and silver in the finished catalysts with the molar mass ratio of copper to silver (Cu:Ag = 1: 0, 0.75: 0.25, 0.5: 0.5, 0.25: 0.75, 0: 1) keeping 1weight % total loading of copper and silver metals on SBA-15 support. In a typical procedure, 1 g the 0.5Cu-0.5Ag/ SBA-15 was prepared by taking 0.99grs of SBA-15, aqueous solution containing 0.0787gr of AgNO3 and 0.19gr of Cu(NO3)2.3H2O. The mixture was evaporated to dryness and the catalyst samples were dried at 100 °C for 12 h and calcined at 450 °C for 6 h. The catalysts are labelled as (a) SBA-15, (b) 1%Cu/SBA-15, (c) 1%Ag/SBA-15, (d) 0.25Cu-0.75Ag/SBA-15, (e) 0.5Cu-0.5Ag/SBA-15 (f) 0.75Cu-0.25Ag/SBA-15. The prepared Cu-Ag/ SBA-15 nanoalloy materials were characterized by low angle XRD, wide-angle XRD, nitrogen adsorption–desorption and SEM techniques.
2.3 Characterization of Cu-Ag/SBA-15 catalysts
The X-ray diffraction (XRD) patterns were recorded at room temperature using a Rigaku, Multiflex, diffractometer with a nickel filtered CuKα radiation of wave length 1.5418 A° at a power of 40 kW and a current of 100 mA in the 2 h range of 0.5–5° and 5–80° for the verification of SBA-15 structural ordering and Cu, Ag phase behavior respectively.
N2 adsorption–desorption isotherms were recorded for catalysts using a Tristar 3000 V 6.08A instrument (M/S Micromeritics Instruments Corporation, USA) at -196 °C. The samples were outgassed at 200 °C for 4 h before the measurement. BET method was used to calculate the surface areas using the amount of N2 adsorbed at -196 °C. The scanning electron microscopy images were recorded using a Hitachi S-520 SEM instrument. ICP-MS studies were made a simultaneous ICP-AES allied analytical system (Perkin Elmer 3100XL).
2.4 Catalytic activity test
Using 0.1 g of catalyst, the activities of each catalyst were examined for control microorganisms in raw water. In a 100 ml sterile transparent tube with a screw cap, 0.1 g of the catalyst was further dipped in 50 cm3 of raw water (pond water, which is rich in microorganisms with E. coli) and agitated for 1 h in batch mode at room temperature. The catalyst was removed after an hour, and the water's bacterial content was quantitatively examined.
2.5 Quantitative analysis of microorganisms
Raw water to be analyzed was taken and serially diluted to 10−6 dilution in a series of six test tubes. One millilitre of the raw water was taken in the first test tube containing 9 ml of saline solution and from it 1 ml was taken into the second test tube also containing 9 ml of saline solution. This process is repeated to the last, i.e., 10−6 dilution. Since the raw water contains large number of microorganisms, which cannot be counted, it is necessary to dilute it. From each test tube 0.1 ml of water was taken and was spread using a spreader, on a Petri-plate containing solidified nutrient agar and were incubated at 310 K for 24 h [17]. This entire process was done in the UV laminar airflow. The number of colonies grown were then counted after incubation.
3 Results and discussion
3.1 Characterization of Cu-Ag/SBA-15 nanoalloy catalysts by low-angle XRD
To assess the structural integrity of the catalysts, low-angle XRD patterns of Cu-Ag/SBA-15 catalysts including SBA-15 were recorded and the resultant patterns are shown in Fig. 1. The XRD patterns of Cu-Ag/SBA-15 nanoalloy show three well-resolved diffraction peaks at about (2θ = 0.89, 1.50, and 1.73º) that are assigned as (100), (110) and (200) reflections, associated with p6mm morphology. These XRD results confirm that hexagonal SBA-15 with a large lattice parameter is thermally stable and mesoscopically well-ordered. In other words, the structure of siliceous SBA-15 has been retained even after loading of Cu and Ag. The unit cell parameter and other structural parameters determined for Cu-Ag/SBA-15 were shown in Table 1.
3.2 Characterization of Cu-Ag/SBA-15 nanoalloy catalysts by wide-angle XRD
To assess the crystalline behavior of Cu and Ag species that are dispersed on the surface of SBA-15 support X-ray diffraction analysis was made in the 2θ range of 5–80 (Fig. 2). A large hump that was observed in all the sample at about 2θ range 20–30 corresponds to SiO2 in mesoporous silica. A small diffraction peak is observed in 1%Cu/SBA-15 catalyst at 2θ = 36.12° might be related to Cu(I). No corresponding metallic peaks of Cu or Ag were observed and a small diffraction peak at 2θ values at 36.27° was observed in all Cu-Ag/SBA-15, which might be indicates the formation of alloy formation.
3.3 Characterization of Cu-Ag/SBA-15 nanoalloy catalysts by N2 adsorption–desorption isotherms
Figure 3 shows the nitrogen adsorption–desorption isotherms for parent SBA-15 and Cu-Ag nanoalloy materials. The isotherms obtained for parent SBA-15 and Cu-Ag nanoalloy materials are of type IV and exhibited a hysteresis loop of H1 type in accordance with the IUPAC classification [19, 20], which indicates that the parent SBA-15 mesoporous structure was well maintained during the stages of catalyst preparation. The isotherms feature with sharp adsorption and desorption branches indicating a narrow meso pore size distribution (Fig. 3). The physico-chemical characteristics of the catalysts have been included in Table 1. It is observed that the decrease in surface area and pore volume of the catalysts after the loading of Cu and Ag on to the SBA-15. The decrease in the surface area and pore volume of the catalysts is due to the pore blockage by the metal nanoparticles.
3.4 Characterization of samples by scanning electron microscopy
Scanning electron microscopic image of 0.25Cu-0.75Ag/SBA-15 has been displayed in Fig. 4. The wormhole-like morphology of parent SBA-15 is well preserved in the 0.25Cu-0.75Ag /SBA-15 nanoalloy material.
3.5 Destruction of microorganisms present in drinking water
To a 50 ml of water containing coliforms, 0.5 g of 1%Cu/SBA-15 catalyst was added and stirred for 1 h, followed by separated the catalyst by a simple filtration. 1 ml of filtrate water that is to be analyzed is taken in a test tube containing 9 ml of saline water followed by a sequential dilution in a series of 6 test tubes each containing 9 ml of saline water. Similarly, 1 ml of raw water is taken in the first test tube containing 9 ml of saline solution and from it, 1 ml is taken into the second test tube also containg 9 ml of saline solution. This process is repeated 6 times from each test tube, 0.1 ml of the water is taken and is spread on the petri-plates using a spreader containing solidified nutrient agar and are incubated at 37 ºC for 24 h. The number of coliforms grown is then counted after incubation.
The microorganism destruction activity of Cu/SBA-15, Ag/SBA-15 and three Cu-Ag/SBA-15 catalysts was displayed in the following Tables. First, the destruction of microorganisms in water was checked with 0.5 g of 1%Cu/SBA-15 and 1%Ag/SBA-15 catalysts individually. From the results depicted in Tables 2 and 3, it is clear that the Ag/SBA- 15 catalyst is better in controlling the microorganisms compare with Cu/SBA-15.
In the subsequent tests examined, the catalytic performance of several Cu-Ag/SBA-15 nanoalloy catalysts were depicted in Tables 3, 4 and 5. No coliforms were found after treating the water with the catalyst 0.25Cu-0.75Ag/SBA-15 at 0.25 g and 0.5 g level. Whereas with two other catalysts with the compositions (0.75Cu-0.25Ag/SBA-15 and 0.5Cu-0.5Ag/SBA-15), a few number of coliforms were detected. This superior activity of the 0.25Cu-0.75Cu/SBA-15 catalyst is due to its high surface area, i.e. 652m2/g. In the case of 0.5Cu-0.5Ag/SBA-15 and 0.75Cu-0.25Ag/SBA-15, the surface area is found 632m2/g and 620 m2/g respectively. It can be seen from the Table 1 that; the surface area of individual catalysts is lower than the surface area of alloy catalysts. Accordingly, the activity of the catalysts in suppressing the coliforms can be seen from Tables 4, 5 and 6.
In summary, a variety of Cu-Ag/SBA-15 nanoalloy catalysts were synthesized using the incipient wetness impregnation method, and their ability to suppress microorganisms in drinking water was evaluated. Among the series of catalysts prepared, 0.25Cu-0.75Ag/SBA-15 catalyst exhibits exceptional activity in controlling the microorganisms. The exceptional activity of 0.25Cu-0.75Ag /SBA-15 catalyst over other Cu-Ag/SBA-15 catalysts can be attributed to its large surface area compared with other catalysts.
Data availability
Data is provided within the manuscript.
References
Malato S, Fernandez-Ibanez P, Maldonado MI, Blanco J, Gernjak W. Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal Today. 2009;147:1.
Chong MN, Jin B, Chow CWK, Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010;44:2997.
Prashant J, Pradeep T. Potential of silver nanoparticle‐coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng. 2005;90:59.
Yaohui LV, Liu H, Wang Z, Liu S, Hao L, Sang Y, Liu D, Wang J, Boughton RI. Silver nanoparticle-decorated porous ceramic composite for water treatment. J Memb Sci. 2009;331:50.
Matilainen A, Sillanpää M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere. 2010;80:351.
Al-Abri M, Al-Ghafri B, Bora T, Dobretsov S, Dutta J, Castelletto S, Rosa L, Boretti A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. npj Clean Water. 2019;2:2
Bodzek M, Konieczny K, Kwiecińska A. Application of membrane processes in drinking water treatment–state of art. Desalinat Water Treat. 2011;35:1.
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662.
Dutta T, Barman A, Bhattacherjee S. Antimicrobial silver nanoparticles for water disinfection: a short review on recent advances. Nanotechnol Environ Eng. 2024;9:111.
Chen M, Chen S. Process for preparing antibacterial antimildew polyacrylic fibers and its filter net for air conditioner. CN Patent 1355335, 2002.
Lee HJ, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38:2199.
Wang QF, Yu HJ, Zhong L, Liu JQ, Sun JQ, Shen JC. Incorporation of silver ions into Ultrathin Titanium Phosphate films: in situ reduction to prepare Silver Nanoparticles and their antibacterial activity. Chem Mater. 2006;8:1988–94.
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679.
Zhang LZ, Yu JC, Yip HY, Li Q, Kwong KW, Xu AW, Wong PK. Ambient light reduction strategy to synthesize silver nanoparticles and silver-coated TiO2 with enhanced photocatalytic and bactericidal activities. Langmuir. 2003;19:10372.
Wang L, He H, Zhang C, Sun L, Liu S, Yue R. Excellent antimicrobial properties of silver-loaded mesoporous silica SBA-15. J Appl Microbiol. 2014;116:1106.
Siva Kumar V, Nagaraja BM, Shashikala V, Padmasri AH, ShakuntalaMadhavendra S, David Raju B, Rama Rao KS. Highly efficient Ag/C catalyst prepared by electro-chemical deposition method in controlling microorganisms in water. J Mol Catal A. 2004;223:313.
Shashikala V, Siva Kumar V, Padmasri AH, David Raju B, Venkata Mohan S, NageswaraSarma P, Rama Rao KS. Advantages of nano-silver-carbon covered alumina catalyst prepared by electro-chemical method for drinking water purification. J Mol Catal A. 2007;268:95.
Reddy KHP, Shashikala V, Anand N, Sandeep C, Raju BD, Ramarao KS. A study on control of microorganisms in drinking water using Ag-Cu/C catalysts. Open Catal J. 2011;4:47–53.
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science. 1998;279:548.
Ganji S, Enumula SS, Marella RK, Rama Rao KS, Burri DR. RhNPs/SBA-NH2: a high-performance catalyst for aqueous phase reduction of nitroarenes to aminoarenes at room temperature. Catal Sci Technol. 2014;4:1813.
Ganji S, Mutyala S, Neeli CKP, Rama Rao KS, Burri DR. Selective hydrogenation of the C[double bond, length as m-dash]C bond of α, β-unsaturated carbonyl compounds over PdNPs–SBA-15 in a water medium. RSC Adv. 2013;3:11533.
Ganji S, Bukya P, Vakati V, Kamaraju Seetha RR, Burri DR. Highly efficient and expeditious PdO/SBA-15 catalysts for allylic oxidation of cyclohexene to cyclohexenone. Catal Sci Technol. 2013;3:409.
Ganji S, Narani A, Kamaraju Seetha RR, Burri A, Park SE, Burri DR. Cu/SBA-15 is an efficient solvent-free and acid-free catalyst for the rearrangement of Benzaldoxime into Benzamide. Catal Lett. 2011;141:1865.
Enumala SS, Varkolu M, Mutyala S, Ganji S, Rama Rao S, Burri DR. Vapor phase esterification of levulinic acid over ZrO2/SBA-15 catalyst. Catal Commun. 2016;75:1.
Neeli CKP, Ganji S, Ganjala VSP, Kamaraju SRR, Burri DR. Oxidative coupling of primary amines to imines under base-free and additive-free conditions over AuNPs/SBA-NH2 nanocatalyst. RSC Adv. 2014;4:14128.
Acknowledgements
The authors are thankful to the Principal, CBIT for his support and CSIR-IICT for research facilities.
Author information
Authors and Affiliations
Contributions
GS: Conceptualization, Investigation, Writing –original draft KR: Validation, Visualization, Methodology = KG: Interpretation of graphs and Writing review RM: Writing review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganji, S., Kola, R., Gullapelli, K. et al. Cu-Ag/SBA-15 nano catalysts for the control of microorganisms in water. Discover Nano 20, 16 (2025). https://doi.org/10.1186/s11671-024-04176-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-024-04176-5
Keywords
Profiles
- Ramesh Kola View author profile