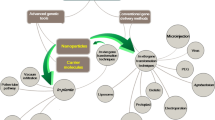
Abstract
Genetic engineering in plants serves as a crucial method for enhancing crop quality, yield, and climate resilience through the manipulation of genetic circuits. A novel genetic transformation approach utilizing nanocarriers as a sound plant genetic engineering technique enables the delivery of DNAs or RNAs into the plant cells. Significant advances have recently been made on the nanotechnology-based delivery of nucleic acids in plants. In this review, several nanoparticle-mediated DNA and RNA delivery systems are discussed respectively, involving latest progresses and drawbacks of these approaches used in plant genetic engineering. We also underscores the current challenges that must be addressed in the implementation of nanoparticles-based strategies for plant gene delivery. Furthermore and more importantly, plant-derived exosome-like nanoparticles that facilitate nucleic acids transfer between organisms was initially proposed as a novel and promising nanodelivery platform for the CRISPR/Cas9 genome editing toolkit in plants. We believe that this review will be beneficial for an effective exploration of nucleic acid nanodelivery to aid the plant genetic engineering in modern agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
It is a great challenge to meet the growing food demands under the circumstance of increasing world population and rapid global climate change [1]. To address this challenge, plant genetic engineering is viewed with high hopes and demonstrates great potential [2, 3]. In general, plant genetic engineering techniques includes transgene, RNA interference (RNAi) and CRISPR/Cas9 genome editing. Transgene is involved heterologous expression of exogenous gene(s) in host plants [4]. RNAi, a post-transcriptional gene silencing (PTGS) process uses small interfering RNAs (siRNAs) to silence target gene(s) [5]. CRISPR/Cas9 employs a single guide RNA (sgRNA) guiding the Cas9 nuclease to cut specific plant genomic DNA fragment(s) [6]. However, effective and convenient approaches to deliver these genetic engineering components into the plant cells are lacking especially for those woody plants, e.g., species of Camellia and Eucommia [7].
The extensively used genetic transformation methods in plants (i.e. Arabidopsis, tobacco, tomato, rice and poplar) include Agrobacterium and virus infection, and particle bombardment (gene gun) [8]. Although widely used, there are still drawbacks to overcome: (1) Agrobacterium and virus vectors are host specific, infecting only sensitive plant species. (2) The viral vector usually achieves transient transfection, and thus unable to be inherited to the offsprings. (3) Agrobacterium and particle bombard methods require the plant regeneration process via tissue culture, which is heavily genotype dependency, time consuming and labor intensive [9, 10]. Therefore, developing efficient and convenient genetic transformation methods is not only a practical need but also will revolutionize the development of plant genetic engineering.
Nanoparticles are widely used in the medical field recently years for their excellent properties of being small-sized (c. 1–100 nm) and large surface areas [10]. Advancements in nanomedicine have paved the way for plant nanobiology, an emerging filed of plant sciences that especially investigates the application of nanotechnology in agriculture [11, 12]. A key focus is the utilization of nanomaterials as carriers for gene delivery in plants, which promises a novel and more efficient approach to plant genetic engineering [13]. During last two decades, many types of nanoparticles have been developed as gene vectors in plants, including carbon-, silicon- and metal-based nanoparticles [14]. In this work, the available plant nanodelivery systems of DNA and RNA are firstly summarized. And then the current challenges that nanoparticles-mediated plant gene delivery faces are discussed. Eventually, a potential and promising natural nanodelivery platform of CRISPR/Cas9 is proposed for plant genetic engineering.
2 Nanoparticle-mediated DNA delivery in plants
In plant genetic engineering, exogenous genes are usually cloned into expression plasmids, such as pCAMBIA-series vectors, for delivering into intact cells to archive transient or stable expression [15]. There are several nanoparticles including magnetic nanoparticles (MNPs), carbon-based nanoparticles, mesoporous silica (MSNs), and peptide-based nanoparticles have been developed as plasmid DNA (pDNA) delivery vehicles (Fig. 1).
2.1 Magnetofection of DNA
Magnetic nanoparticle-based transformation (magnetofection) employs magnetic forces to deliver complexs of magnetic nanoparticles and DNAs (MNPs-DNAs) into cells, which has gained popularity in animal systems [16]. This technique has also been adapted to plants recently. Hao and colleagues firstly demonstrated the applicability of MNPs as a gene carrier for plant cells [17]. They synthesized magnetic gold nanoparticles (mGNPs) with diameters ranging from c. 20–30 nm and functionally modified mGNPs surface by adding free amino groups from polyethylene glycol (PEG). Fluorescein isothiocyanate (FITC) was conjugated to the mGNPs, which were then delivered into canola (Brassica napus) protoplasts using an external magnetic field. A high delivery efficiency of up to 95% was reported for FITC-labelled mGNPs. Additionally, plasmids encoding GUS reporter gene were covalently bound to the surface of mGNPs and also delivered into canola protoplasts and walled cell suspensions leading to transient GUS expression. Magnetofection could also be used for transformation of tobacco mesophyll protoplasts [18]. Specifically, the plasmid pCAMBIA1303 was delivered by polyethyleneimine (PEI) modified Fe3O4 MNPs resulting in transient GUS gene expression within the protoplasts.
Zhao et al. [19] further developed a simple, rapid and efficient pollen magnetofection technology to generate transgenic seeds, thereby bypassing the labor-intensive and time-consuming tissue culture process. In this method, Fe3O4 MNPs were positively charged and coated with PEI to bind negatively charged pDNAs through electrostatic attraction. The MNP/DNA complexes were then mixed with pollens and guided by an external magnetic field to direct them into the pollen via germination apertures. The transformed pollens were then used for artifical pollination and the resulting seeds were sown to screen for transgenic plants. The authors selected cotton (Gossypium hirsutumcotton) as a model species to test this technique, successfully obtained transgenic cotton plants. In addition, they further demonstrated the versatility of pollen magnetofection using the pBI121 GUS plasmid as a reporter in four other flowering plants: three eudicots- cocozelle (Cucurbita pepo), pepper (Capsicum annuum) and pumpkin (Cucurbita moschata), and one monocot- lily (Lilium brownii). Transgenic pepper and pumpkin plants were also obtained with the GUS gene integrated into the host genomes. This pioneering work combined magnetofection with pollen-mediated genetic transformation, offering a tissue culture-free and host-independent approach that may be applicable for genetic transformation in a wide range of plant species.
Recently, pollen magnetofection technology has successfully been applied to achive efficient genetic transformation in maize (Zea mays) [20]. Plasmid DNA encoding red fluorescent protein (RFP), enhanced green fluorescent protein (EGFP), GUS or bialaphos resistance (bar) was successfully delivered into pollen grains for six inbred lines of maize (178, B73, HZ178, Jing2416, Jing92 and Zheng58). Expression of EGFP or GUS was detected in 5–16% of T1 transfected seedlings, while glufosinate resistance was observed in 1.41% of T1 plants transformed with the bar gene. Notably, pretreating maize pollens at 8 ℃ for 10 min prior to magnetofection is a critical step that significantly enhances the transformation efficiency.
Moreover, the pollen magnetofection system has also been successfully employed for the genetic transformation of tree peonies (Paeonia ostii and P. rockii) using GUS gene as the reporter [21]. Additionally, the plasmid vector pYBA1132 containing EGFP gene was also delivered into the protoplasts of a green algae (Chlorella ellipsoidea) resulting in a transient expression of EGFP [22]. Notably, this technology has also been applied to deliver CRISPR/Cas9 plasmid for genome editing in pepper [23]. The presence of CRISPR/Cas9 pDNA was reported in both T0 and T2 generation plants, suggesting the potential of pollen magnetofection for precise genome editing.
2.2 Carbon-based nanocarriers of DNA
Carbon-based nanoparticles, including carbon nanotubes (CNTs), carbon dots (CDs), graphene, nanodiamond, etc., exhibit exceptional electrical, optical, mechanical and thermal properties [24]. CNTs are elongated, cylindrical nanoparticles characterized by a high aspect ratio and are typically categorized into two types: single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs) [25]. SWCNTs are small diameter sized (1–3 nm), whereas MWCNTs are larger (5–40 nm). CDs, another class of carbon nanomaterials, are measured in c. 5–10 nm and noted for their excellent biocompatibility [26]. Both CNTs and CDs by far have been developed as nanocarriers for delivering foreign DNAs in plant cells.
2.2.1 Carbon nanotubes
Liu et al. [27] firstly used oxidized SWCNTs (o-SWCNTs) to archive DNA delivery into intact Bright Yellow (BY-2) cells of Nicotiana tobacum, demonstrating the potential of SWCNTs as gene nanotransporters capable of penetrating the plant cell wall and cell membrane. Following this, Burlaka and colleagues [28] advanced the application of CNTs in plant genetic transformation. They used commercial SWCNTs and MWCNTs as nanocarriers to deliver the pGreen0029 plasmid into callus cells, mesophyll protoplasts and leaf explants of N. tabacum. Their results showed transient expression of the YFP reporter gene in the protoplasts using both SWCNTs (20 µg/mL) and MWCNTs (15 µg/mL). Notably stable expression of the nptII gene was only seen in the regenerated plants derived from N. tabacum callus and leaf explants when using SWCNTs, not MWCNTs. This study underscored the suitability of SWCNTs-based nanocarriers for genetic transformation in both plant protoplasts and walled cells. Subsequently, Golestanipour et al. [29] assessed the efficiency of the arginine functionalized SWCNTs (Arg-SWCNTs) for gene delivery in plants. They suggested that the cylindrical nanostructure and cationic groups of engineered Arg-SWCNTs facilitated the transfer of pDNAs into tobacco (N. tabacum) root cells. Successful expression of GFP was confirmed through fluorescence microscopy and western blotting analysis.
Remarkably, Demirer et al. [30] and Kwak et al. [31] successfully employed polymer-functionalized SWCNTs to deliver genes into intact plants without external assistance. Demirer and colleges [30] utilized carboxylic acid-functionalized SWCNTs as raw materials, covalently attaching PEI to these nanotubes to impart a net positive charge, resulting PEI-SWCNTs. The negatively charged GFP-encoding plasmids were subsequently attached to the PEI-SWCNTs via electrostatic attraction. This system enabled efficient DNA delivery into mature leaves of three eudicots species- N. benthamiana, G. hirsutum and Eruca sativa, and one monocot species- Triticum aestivum. Robust GFP expression was detected at both mRNA and protein levels, though no integration of the transgene into the host genome was detected. This method achieved high transformation efficiency reaching up to 85% in the arugula protoplasts. Furthermore, Kwak and colleagues [31] provided evidence of gene delivery using SWCNTs as nanocarrier in mature N. tabacum, E. sativa, Nasturtium officinale and Spinacia oleracea plants, as well as in the isolated A. thaliana protoplasts. They used chitosan, an alternative cationic polymer to covalently modify SWCNTs (CS-SWCNTs), achieving chloroplast-selective gene delivery. It was proposed that the acidic pH value of plant cell cytosol (~ 5.5) facilitates the binding of pDNAs to CS-SWCNTs, whereas the alkaline pH value of chloroplast (~ 8) weakens the electrostatic interactions between pDNAs and CS-SWCNTs, leading to the release of pDNAs from CS-SWCNTs.
Following the above breakthroughs, Lew et al. [32] further synthesized imidazolium-functionalized SWCNTs (IM-SWCNTs) and efficiently delivered the GFP reporter gene into oil palm (Elaeis guineensis) pollens without external physical aid. IM-SWNTs displays enhanced biocompatibility with oil palm pollens compared to the previously used PEI-SWCNTs [30] and CS-SWCNTs [31]. The study highlighted the zeta potential of IM-SWNTs as a critical factor influencing pollen transformation efficiency. Moreover, Ghaghelestany et al. [33] explored the application of PEI-SWCNTs for gene transformation in German chamomile callus cells, revealing that combining ultrasound and PEI-SWCNTs markedly improved the transformation efficiency.
2.2.2 Carbon dots
Wang et al. [34] firstly demonstrated that CDs are a promising vehicle to deliver foreign DNAs into intact plants. They synthesized CDs using an electrochemical etching method and subsequently modified these CDs with PEI to create a positively charged nanocomposite (CDP). This CDP was used to successfully deliver two pDNA vectors i.e. pJIT163-mCherry and pJIT163-hGFP into the leaves of rice (Oryza sativa), wheat (T. aestivum) and mung bean (Phaseolus radiatus), as well as into rice roots. Furthermore, the CDP delivery system was effective in transforming rice callus cells achieving both hygromycin resistance and GUS expression. The study suggested that the CDP protects DNA from DNase degradation and facilitates its transport into plant cells, thereby enhancing the efficiency of genetic transformation.
Notably, a straightforward method for spray-on gene editing was developed for plants in the preprint paper of Doyle et al. [35]. The researchers prepared PEG functionalised CDs (PEG-CDs) and conjugated pDNAs to these nanoparticles via electrostatic attraction. Thanks to their innate fluorescence at 475 nm, the CDs are easily tractable suggesting their diffusion throughout the somatic cells, but not in the chloroplasts. The GFP expression was observed by directly spraying the pDNAs/PEG-CDs formulations onto leaves of four monocots: wheat, maize, barley and sorghum. Furthermore, the study successfully delivered a CRISPR/Cas9 plasmid expressing Cas9 protein and sgRNAs targeting two regions of the SPO11 gene into wheat leaves, achieving a deletion mutation in the SPO11 gene. This approach enabled CRISPR/Cas9-mediated gene editing in cereal crops without integrating exogenous DNA into the host genome.
2.3 Peptide-based nanocarriers of DNA
Peptides with approximately 30 amino acids in length have been employed as nanocarieers to transfer DNA and protein into animal cells [36]. To date, numerous cell-penetrating peptides (CPPs) have also been used for gene delivery in plant cells for their native cell wall/membrane penetrating capability. These polycationic peptides can encapsulate negatively charged DNAs, protecting them from enzymatic degradation and facilitating their delivery into plant cytoplasm and specific organelles, e.g. mitochondria and chloroplasts [37].
In 2004, Rosenbluh and colleagues [38] demonstrated for the fist time that histone peptides could penetrate the plasma membrane of petunia (Petunia hybrida) protoplasts. Following this seminal work, arginine-rich intracellular delivery (AID) peptides were subsequently used to efficiently deliver GFP protein into onion epidermal and root tip cells, tomato roots and tobacco BY-2 cells [39]. The CPP of TAT2 was also developed to archive high transient GUS gene expression in the wheat immature embryos [40]. Moreover, a peptide-based gene delivery system was designed by combing CPPs (such as BP100 or TAT2) with a polycation (like R9 or KH9) to deliver pDNA into intact leaf cells of A. thaliana and N. benthamiana [41]. Transient GFP expression was observed in the cytoplasm using this composite peptides transporter. These studies collectively demonstrated that peptides serve as efficient cell-penetrating agents for delivering exogenous DNA into intact plant cells.
Notably, Chuah et al. [42] were the first to achieve plant mitochondria transfection using peptides-based nanocarriers. They combined CPP with mitochondria-targeting peptide (MTP) to form CPPKH-MTPKH-pDNA complexes. This approach led to observable transient GFP expression in the mitochondria of A. thaliana leaves in a short time of 12 h of transfection. Furthermore, the same research group created stable mitochondrial transgenic lines of A. thaliana and N. tabacum in 2018 [43], where exogenous genes were integrated into the host mitochondrial genomes. More recently, Thagun et al. [44] developed a technique for transient plastid transformation using peptide-based gene carrier. They formulated peptide/pDNA complexes by integrating a CPP with a chloroplast-targeting peptide (CTP). These complexes enabled efficient delivery of pDNA into plastids through simple leaf infiltration, offering a valuable tool for plastid genetic engineering.
2.4 Silicon-based nanocarriers of DNA
Silicon is considered as one of the beneficial elements for plants and silica-based nanoparticles have been utilized to enhance plant tolerance to abiotic stresses [24]. In 2007, Torney and colleagues firstly demonstrated that MSNs could serve as effective nanocarriers for pDNA in plants [45]. In their study honeycomb gold-capped MSNs with 3 nm pores were designed, which successfully delivered a GFP-encoding plasmid into intact leaves of N. tabacum by gene gun. Subsequently, Martin-Ortigosa et al. [46] showed that gold-plated MSNs with a large pore diameter of 10 nm could co-deliver pDNA and protein to the epidermal tissues of Allium cepa. Notably, gold-plated MSNs were also used to deliver site-specific recombinase, such as the Cre protein, for gene editing in maize [47]. However, it is important to note that this gold-modified MSNs delivery system still require the use of an expensive gene gun to penetrate the cell wall, highlighting a limitation in terms of cost and accessibility.
Furthermore, Chang et al. [48] developed organically functionalized MSNs measuring 40–50 nm and successfully delivered pDNA-mCherry into intact roots of A. thaliana. This modified MSN-based gene delivery system can passively penetrate the cell wall without the aid of gene gun and has a high transformation efficiency (up to 46.5%). Recently, aminopropyl-triethoxysilane functionalized MSNs (APTES-MSNs) with a similar size range of ~ 40 nm were synthesized and used to deliver pDNA-GUS into tomato leaves and shoots via simple spray or injection [49]. Successful transfer of the resistance gene cry1Ab of Tuta absolutawas was also accomplished in tomato plants by APTES-MSNs. These studies demonstrate that MSNs can deliver pDNA into plant tissues without external assistance, providing a promising direction for modifying MSNs as efficient tools for gene delivery in plants.
Based on the comparison of aforementioned nanodelivery systems of DNAs, it is evident that significant refinement of existing methodologies is required to suit specific plant species. For instance, Vejlupkova et al. [50] observed no transient expression of pDNA-GFP in three monocot species (lily, maize, and sorghum) when using pollen magnetofection system developed by Zhao et al. [19]. Two key factors likely contribute to these discrepancies:
Pollen structure variability Pollen strucuture varies significantly among different plant species. Pollens of lily, maize and sorghum used in Vejlupkova et al. [50] are monosulcate, whereas pollents of cotton, pepper and pumpkin examined by Zhao et al. [19] are tricolpate. Monosulcate pollens may present greater challenges for transformation compared to tricolpate pollens [19, 50].
Pollen germination aperture size The size and state of the pollen germination aperture can also influence the transformation efficiency. Wang et al. [20] found that maize pollens collected from greenhouse-grown plants were often capped with an operculum, which prevented the entry of MNP-DNA complexes. In contrast, pollens collected from field-grown maizes, after low-temperature (8 °C, 10 min) pretreatment, turned to be open and could be successfully transformed.
3 Nanoparticle-mediated RNA delivery in plants
RNAi is a potent tool in plant genetic engineering for regulating the expression of endogenous genes [13, 51]. Commonly, in the RNAi pathway, Dicer-like proteins (DCL) cleave double-stranded (dsRNA) into siRNAs sized with 21–22 bp. These siRNAs are then incorporated into RNA-induced silencing complex (RISC) which further degrades the target mRNA or inhibits its translation [5, 14]. Traditionally plant RNAi involves coding siRNAs into DNA vectors, which are then delivered into plant cells using the tobacco rattle virus (TRV). However, this approach is limited to certain crops and can be challenging to apply broadly [52]. Recently, advancements in nanotechnology have introduced various nanocarriers for delivering siRNA directly into plant cells for gene silencing (Fig. 2), expanding the scope of RNAi applications.
3.1 Carbon-based nanocarriers of RNA
Demirer et al. [53] revealed that intracellular transport and protection of siRNAs from degradation are crucial for the effectiveness of SWCNTs-based siRNA delivery platform in plants. Their study demonstrated that SWNTs could effectively carry Cy3-RNA into N. benthamiana leaves via needleless syringe infiltration. This method successfully silenced both the GFP transgene and an endogenous gene ROQ1 using corresponding siRNA/SWCNTs complexes. Notably, leaves infiltrated with the siRNA/SWNTs complex showed a reduction of over 90% in the GFP mRNA transcript levels in 24 h and one single infiltration maintained this silencing effect for up to three days. This underscores the potential of SWCNTs as robust carriers for siRNAs delivery.
Moreover, Schwartz et al. [54] demonstrated successful delivery of siRNA into N. benthamiana and Solanum lycopersicum using CDs. In this study, GFP transgene was effectively repressed through a simple low-pressure spraying method, with no need of leaf infiltration. Additionally, two endogenous genes, CHLH and CHLI were also knocked-down. When comparing silencing efficiency, the researchers found that CDs produced from 5kD branched PEI showed the highest silencing efficiency (> 90%). This work suggests the potential of CDs as efficient nanocarriers for RNAi applied in plants, offering an non-invasive and highly effective method for gene knock-down.
Polymer-functionalized graphene oxide nanoparticles (GONs) were also developed to deliver siRNA into N. benthamiana [55]. Sheet-like GONs were fabricated using PEI and PEG, and loaded siRNA to form a near-spheroidal GONs-siRNA complex with sizes ranging from 15 to 50 nm. The GONs-siRNA formulation exhibited a remarkable silencing efficiency of 97.2% in intact leaves within 24 h of treatment. This high efficiency likely attributed to the enhanced ability of the GONs to penetrate cell walls in addition to efficient cellular uptake of the siRNAs. GONs appears to play critical roles in facilitating both penetration and absorption, largely improving the efficiency of gene silencing.
3.2 Silicon-based nanocarriers of RNA
More recently, an advanced multi-gene silencing nanodelivery platform has been developed using MSN-siRNA spraying [56]. This novel delivery method enables efficient siRNAs uptake in N. benthamiana leaves via topical application, eliminating the need for mechanical methods such as infiltration. The silencing efficiency achieved was remarkable, reaching up to 98% at the molecular level for GFP transgene. Additionally, this platform successfully silenced five endogenous genes i.e. PDS, ChlH, SOS, HHL1 and FtsH2 leading to observable phenotypic changes. Notably, MSNs were capable of concurrently delivering siRNAs targeting FtsH2, HHL1, SOS and GFP, resulting simultaneous multi-gene silencing within the leaves. Moreover, sustained gene silencing lasting for 14 days was detected following sequential deliveries of siRNAs targeting SOS, FtsH2, HHL1 and GFP genes.
The MSN-siRNA delivery platform offers a versatile approach to enhance seed shattering resistance in wild rice (O. alta) [57]. Foliar spraying of MSN/siRNA complexes resulted in up to 70% gene silencing for the endogenous gene PDS and 75% silencing for the Ruby transgene. Furthermore, four candidate genes (SH01-SH04) of O. alta were analyzed by using the MSN-siRNA gene silencing technique, resulting that SH01, SH03 and SH04 were shattering genes of O. alta. This study showed the pivotal role of MSN-based siRNA delivery in elucidating gene functions in non-model plants.
3.3 Gold-based nanocarriers of RNA
Gold nanoparticles (AuNPs) were synthesized and functionalized by 25kD branched PEI, resulting in PEI-AuNPs nanoparticles [58]. These nanoparticles could efficiently integrate siRNAs to form stable formulations capable of delivering siRNAs into Arabidopsis cells. An 80% expression reduction of NPR1 gene was achieved using the AuNPs-siRNANPR1 formulation. As anticipated, Columbia-0 Arabidopsis plants treated with AuNPs-siRNANPR1, exhibited yellow leaves, similar to the phenotype observed in npr1 mutants.
Furthermore, PEI-functionalized gold nanoclusters (PEI-AuNCs) were developed for siRNA delivery in plants [59]. PEI-AuNCs could effectively protect siRNAs from RNase degradation and and facilitate its passage through the plant cell wall. Efficiency of gene knockdown carried using PEI-AuNCs exceeded 76% for both GFP and ROQ1 genes in N. benthamiana. Notably, the size and shape of gold AuNPs significantly influence the delivery efficiency of siRNAs [60]. In general, smaller and rod-shaped AuNPs are internalized into plant cells more efficiently. The reductions in GFP expression were as follows: 8% for 20 nm gold nanospheres, 29% for 15 nm gold nanospheres, 42% for 5 nm gold nanospheres, 99% for 10 nm gold nanospheres and 39% for gold nanorods. These results suggested that 10 nm spherical AuNPs are the most efficient for siRNAs delivery. Interestingly, while the AuNPs assist in delivering siRNAs to the cell wall, it is only the siRNAs that enter the plant cell, leaving the AuNPs outside the cell membrane.
3.4 LDH-based nanocarriers of RNA
Layered double hydroxide (LDH) nanosheets belong to a family of bio-compatible and degradable clay materials that characterized by their layered structure [61]. The positively charged hydroxide layers make LDH nanosheets an ideal carrier for RNAs delivery. In 2017, Mitter et al. [62] demonstrated that LDH nanosheets measuring 48 nm in diameter and 5.3 nm in thickness successfully loaded dsRNA to form dsRNA/LDH complexes which they named as BioClay. The dsRNA within BioClay exhibited resistance to nuclease degradation and remained stable on the leaf surface for up to 20 days following a single spray application. Importantly, topical application of BioClay protected N. tabacum from CMV virus infection via RNAi pathway. Furthermore, Yong et al. [63] using S. lycopersicum pollen as a model system found that LDH nanosheets with a diameter of 50 nm could internalize dsRNA into pollen within 2–4 h of incubation. The functional dsRNAs delivered by LDH efficiently induced RNAi of the reporter gene GUS, leading to an 89% reduction in mRNA levels.
3.5 DNA-based nanocarriers of RNA
Based on base-pairing principles, DNA nanostructures were synthesized with precisely programmed sizes and shapes, such as a 2.4 nm tetrahedron and 16 nm hairpin-tile monomer [64]. These DNA nanostructures can deliver siRNA into plant leaves without external assistance. Using transgenic mGFP5 N. benthamiana plants as a model, leaves infiltrated with the DNA-nanostructures/siRNA complex showed over a 40% reduction in GFP fluorescence intensity compared with untreated leaves. Notably, the gene silencing efficiency was influenced by the size, shape, compactness, and stiffness of DNA nanostructure.
3.6 Peptide-based nanocarriers of RNA
In addition, a fusion peptide KH9-BP100 with the amino acid sequences of KHKHKHKHKHKHKHKHKHKKLFKKILKYL has been developed as an efficient siRNA carrier into plant cells [65]. This siRNA/peptide complex was positively charged and 100–300 nm in diameter. When infiltration of the complex into intact A. thaliana leaves, RNAi of transgene YFP and endogenous gene CHS was observed, respectively. Recently, the KH9-BP100 peptide was fused with CTP (OEP34) and successfully delivered siRNA into chloroplast [66]. Spray of siGFPS1/KH9-OEP34/BP100 complex onto a transplastomic N. tabacum leaded to an efficient gene knockdown of eGFP.
As summarized in Table 1, a great variety of nanoparticles can successfully deliver siRNA into plant cells compared to pDNA. This disparity may primarily stem from differences in cargo size. siRNA molecules, typically 21–22 bp in size, are considerably smaller than pDNAs that usually range from c. 5000 to 10,000 bp. The diminutive size of siRNAs enhances their ability to penetrate the troublesome barriers of cell wall and cell membrane [9, 36]. In addition, the distinct mechanisms by which siRNAs and pDNAs work in plant cells likely affect their respective nanodelivery success rates. siRNAs are destined for the cytoplasm where they induce degradation of target mRNAs via the RNAi pathway [5]. In contrast, pDNAs have to experience a more intricate journey before performing their functions: they first enter the nucleus for transcription and then proceed to the cytoplasm for translation [4]. Failure at transcription or translation stage can result in the silencing of the reporter gene.
MSNs mesoporous silica nanoparticles, MNPs magnetic nanoparticles, SWCNTs single-walled carbon nanotubes, CDs carbon dots, GONs graphene oxide nanoparticles, LDH layered double hydroxide, CPP cell-penetrating peptide, CTP chloroplast-targeting peptide, AuNPs gold nanoparticles, AuNCs gold nanoclusters, pDNA plasmid DNA, siRNA small interfering RNA.
4 Challenges of using nanoparticles for plant genetic engineering
As previously reported, the utilization of nanotechnology in various aspects of agricultural production has demonstrated promising benefits [3, 11]. Whereas, the development of nanodelivery system for nucleic acids in plant genetic engineering is still in its infancy and faces numerous challenges.
4.1 Phytotoxicity and loading capacity of nanoparticles
The studies mentioned above predominantly utilized artificially manufactured nanomaterials as vehicles for nucleic acids (Table 1). However, recent observations have highlighted the potential inhibitory effect of artificial nanoparticles e.g., CNTs and CDs on plant growth [25, 26]. For example, in studies on rice, MWCNTs were found to exert toxic effect on rice suspension culture, inducing ROS accumulation and oxidative stress in cells [68]. In maize, exposure to CDs at concentrations of 1000 and 2000 mg/L for four weeks resulted in significant reduction in fresh shoot weights by 38% and 72%, and root weights by 57% and 68% [69]. Similarly, CDs with concentrations exceeding 125 mg/L significantly reduced root elongation in A. thaliana, and comparative transcriptome analysis showed that 715 genes in roots were up-regulated following treatment of 1000 mg/L CDs [70].
It is worthnoting that there may be a point of contradiction in the phytotoxicity and loading capacity due to the concentration dependency [71]. To minimize phytotoxicity, concentration of nanoparticles should ideally be at a lower dose [25], however higher concentrations are necessary to load an adequate amount of DNAs or RNAs [56]. For instance, when using Fe3O4 MNPs as a DNA carrier system, it was found that a mass ratio of pDNA to Fe3O4 MNPs ranging from 0.1:1 to 1:1 (w/w) was optimal for pDNA loading [19]. Nevertheless, Fe3O4 MNPs at such concentrations showed a potential phytotoxic effects to cotton, likely leading to a low efficiency of gene transformation [72].
Moreover, it is important to understand the maximum length of DNA that can be effectively delivered by nanoparticles [73]. To date, PEI-SWCNTs have successfully delivered DNA fragments ranging from 4.2 to 5.4 kb into plant cells [30]. Additionally, CS-SWCNTs have facilitated the transfer of larger plasmids of up to 8.8 kb in size [31]. However, the upper-limit for the maximum plasmid size that can be delivered by nanoparticles remains undetermined. Loading pDNA as large as 15 kb onto nanoparticles is challenging and still debatable [9]. To our knowledge, there are scarce reports on nanocarrier-mediated CRISPR-Cas9 genome editing in plants. One main reason for this gap is that DNA plasmids encoding for the CRISPR-Cas9 system are approximately 15 kb, substantially larger than the pCAMBIA-series reporter plasmids (c.10 kb) that are commonly used in proof-of-principle nanodelivery studies. In the future, it is imperative to optimize existing nanodelivery systems to accommodate larger CRISPR-Cas9 pDNA in plant genetic engineering.
4.2 The uptake and translocation of nanoparticles
The cell wall poses a primary barrier to the delivery of nucleic acids into plant cells due to its limited pore sizes (5–20 nm) [74]. In canonical scenario, nanocarrier-DNA or nanocarrier-RNA complexes go through the cell wall and membrane to reach the cytoplasm where they release their cargoes. However, the mechanism by which nanoparticles enter plant cells remains poorly understood [75, 76]. Theoretical and experimental studies on mammals suggested that nanoparticles internalization occurs via lipid membrane exchange [77]. Recent studies on plants suggested that similar mechanism may also be involved in nanoparticle uptake by plant cells [31]. Besides, energy-dependent endocytic pathways, channel-mediate transport and pore formation may play roles in the cellular internalization in plants [7].
Moreover, a recent study by Zhang et al. [60] reported an unusual scenario in which AuNPs did not need to enter cell to deliver siRNA. In this case, AuNPs intercalated with individual plant cell walls, protecting the siRNA cargo from nuclease degradation. Although AuNPs per se was not internalized by the cells, they served as reservoirs for siRNA to provide more opportunity for siRNA to enter plant cells. The uptake mechanism may vary depending on the type of nanoparticles and plant species involved [78]. Therefore, considerable effort is needed to investigate the interactions between nanoparticles and plant cells. Such research will help to guide design strategies for nanoparticle-based gene delivery in plants.
To date, the translocation of nanomaterials between plant cells, tissues and organs remains poorly understood. Both phloem and xylem are likely involved in nanoparticle distribution throughout the plant [76, 79], which is particularly concerning for the development of RNAi-based pesticides. For pests that feed on phloem sap, such as aphids, it is optimal to use nanoparticles that transport through the phloem as dsRNA nanocarrier. Conversely, nanoparticles that travel through xylem are suitable for insects that feed on xylem-sap [80]. In addition, when nanocarrier-DNA or nanocarrier-RNA solutions are sprayed onto leaves they may be transported to flowers, seeds and fruits, potentially leading to unexpected accumulation of nanoparticles in the food chain [81]. This poses potential risk to biodiversity, an issue that has been largely overlooked. For example, high concentrations of Fe3O4 MNPs (10.0 mg/kg) could accumulate in mycorrhizal fungi and destroy the mutualistic symbiosis between fungi and plants [82]. The accumulation results in a significant reduction in mycorrhizal infection rate, lower levels of glomalin-related protein in the soil and decreased alkaline phosphatase activity. Therefore, it is crucial to address the issue of nanoparticles accumulation in non-target organisms during cargo delivery to mitigate these risks.
5 Plant-derived exosome-like nanoparticles as natural nanodelivery platform for CRISPR/Cas9 toolkit
Plant-derived exosome-like nanoparticles (PELNs) are plant-secreted small extracellular vesicles, characterized by a bilayer phospholipid membrane and typically measuring between 30 and 300 nm in diameter [83]. These nanovesicles are similar to animal exosomes and facilitate inter-cellular and inter-species communications through the transport of proteins, lipids and nucleic acids. Owning to their biocompatibility, low immunogenicity, and natural origin, PELNs present promising prospects as drug delivery systems [84]. Previous studies have showed the capability of PELNs to convey therapeutic nucleic acids into mammalian cells, offering potential treatment of various diseases [85]. Likewise, PELNs are probably used as natural nanocarriers for nucleic acids delivery in plant cells. Despite of this, exploration of PELNs as gene delivery system within plants remains to be investigated.
The pioneering work by Kim et al. [86] demonstrated that cancer-derived exosomes mediated CRISPR/Cas9 delivery in human cells. Through electroporation (1000 V, 10 ms, 2 pulses), they successfully encapsulated Cas9-/sgRNA-expressing plasmids within exosomes isolated from HEK293 and SKOV3 cells. This approach led to the suppression of the target gene PARP-1, resulting apoptosis in ovarian cancer. Since then, exosome-liposome hybrid nanoparticles were developed to deliver CRISPR-Cas9 encoded plasmid vectors into the mesenchymal stem cells, achieving knocked-out of the target gene hCTNNB1 [87]. Additionally, exosomes isolated from HEK293T cells were used to carry CRISPR/Cas9 pDNA using commercial transfection reagents Exo-Fect [88]. These exosomes encapsulating genome editing machinery were cable of being absorbed by pancreatic cancer cells, thereby enabling editing of the oncogenic allele KrasG12D. Recently, serum-derived exosomes have been shown to effectively deliver CRISPR-Cas9 ribonucleoprotein (RNP) for modifying the dystrophin gene hDMD [89]. By incorporating CRISPR-Cas9 RNP into exosomes via electroporation, researcher formed EV@RNP complexes to realize cardiac-specific genome editing [90]. Furthermore, exosomes loaded with CRISPR-Cas9 RNP have been employed to inhibit herpes simplex virus1 infection too [91], highlighting the versatility and potential of exosome-mediated delivery system in various therapeutic applications.
In recent studies on plant-pathogen communication, cross-kingdom RNA trafficking by exosome-like extracellular vesicles was observed as well [92, 93]. Notably, the fungus Botrytis cinerea that causes grey mould in hundreds of crops can transport siRNAs into its host plant A. thaliana [94]. These transferred siRNAs further utilized host RNAi machinery to silence immunity-relevant genes of A. thaliana. Fascinatingly, this siRNAs exchange is bidirectional, that is A. thaliana also send siRNAs to B. cinerea to silence its virulence-related genes [95]. In addition, A. thaliana also used exosome-like extracellular vesicles to deliver mRNAs into B. cinerea, leading to the production of proteins within the fungus that reduce its pathogenicity [96]. Moreover, PELNs isolated from citrus juice showed the capability to package exogenous dsRNAs [97]. For instance, citrus PELNs loaded with dsCrcB and dsFUM21 inhibited the expression of Penicillium italicum CrcB gene and Fusarium proliferatum FUM21 gene, respectively. In a study conducted by our group, siRNA-loaded turmeric PELNs were shown to induce the silencing of GFP transgene in N. benthamiana (data unpublished).
Collectively, the above findings suggest that PELNs hold significant promise as a next-generation nanocarrier of CRISPR/Cas9 toolkit to advance genomic editing in plants (Fig. 3). It is probable to generate heritable genetic variation via utilizing PELNs to introduce CRISPR/Cas9 expression pDNA into plant seeds, buds or germ cells such as pollens and ovules. Such applications could accelerate the development of new crop varieties with desirable agronomic traits [98, 99]. Furthermore, PELNs also can serve as carriers for siRNAs, enabling the modulation of genetic circuits that control flowering time, vernalization requirement and drought tolerance [100]. This is beneficial for transient reprogramming of agronomic performance in existing crop varieties, providing a valuable tool to tackle the inevitable extreme weather conditions under global climate change.
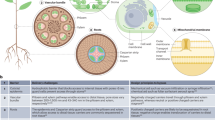
Plant-derived exosome-like nanoparticles (PELNs) mediated delivery of CRISPR/Cas9 plasmid DNA to achieve gene knock-out in plants. During this process, double-strand break of genomic DNAs generated by Cas9 protein is commonly repaired by non-homologous end joining (NHEJ), leading to loss-of-function mutation. If donor DNAs provide the repair template, then the genome can undergo homologous recombination repair (HDR) to realize DNA replacement
6 Conclusion and outlook
As discussion above, the intersection of nanoscience and plant science has promoted the development of plant nanobiology in recent years. The use of nanoparticles for the delivery of DNAs and RNAs in plants is rapidly advancing plant functional genomics and genomic engineering by overcoming limitations associated with traditional methods, such as Agrobacterium-mediated transformation, viral vectors and particle bombardment. Certain nanocarriers can even selectively deliver foreign DNAs and RNAs to chloroplast via simple foliar spray, this not only simplifies the process but also enhances the efficiency and specificity of plastid genetic engineering.
Despite rapid advances in nanodelivery of nucleic acids to plants, several challenges remain to be addressed. Most notably, there is a gap in nanoparticle-mediated genome editing in plants, because the majority of nanodelivery studies have focused on small-sized pDNA and siRNA molecules (20–10,000 bp), with limited explorations into more complex genomic editing tools. During the long-term co-evolution, plants have evolved a mechanism to use PELNs for the trafficking of mRNA and siRNA between organisms. Similarly, fungal cells can secret siRNA-loaded exosome-like nanoparticles into the extracellular space, which further entered plant cells to regulate target gene expression. Moreover, exosomes have been successfully employed to mediate the delivery of CRISPR/Cas9 pDNA or RNP complex for genome editing in human cells. Given these advancements, we propose to develop PELNs as biocompatible and efficient agent to introduce genomic editing tools into plant cells, thereby facilitating precise genetic modifications. Therefore, future research should focus on optimizing PELNs as vehicles for CRISPR/Cas9 components, potentially revolutionizing plant genetic engineering.
Data availability
No datasets were generated or analysed during the current study.
References
Zhao L, Bai T, Wei H, Gardea-Torresdey JL, Keller A, White JC. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nat Food. 2022;3:829–36.
Hofmann T, Lowry GV, Ghoshal S, Tufenkji N, Brambilla D, Dutcher JR, Gilbertson LM, Giraldo JP, Kinsella JM, Landry MP. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat Food. 2020;1:416–25.
Zhang P, Guo Z, Ullah S, Melagraki G, Afantitis A, Lynch I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat Plants. 2021;7:864–76.
Demirer GS, Landry MP. Delivering genes to plants. Chem Eng Prog. 2017;113:40–5.
Chen X, Rechavi O. Plant and animal small RNA communications between cells and organisms. Nat Rev Mol Cell Biol. 2022;23:185–203.
Sandhya D, Jogam P, Allini VR, Abbagani S, Alok A. The present and potential future methods for delivering CRISPR/Cas9 components in plants. J Genet Eng Biotechnol. 2020;18:25.
Wu H, Li Z. Nano-enabled agriculture: How do nanoparticles cross barriers in plants? Plant Commun. 2022;3:100346.
Yan Y, Zhu X, Yu Y, Li C, Zhang Z, Wang F. Nanotechnology strategies for plant genetic engineering. Adv Mater. 2022;34:2106945.
Demirer GS, Silva TN, Jackson CT, Thomas JB, Ehrhardt WD, Rhee SY, Mortimer JC, Landry MP. Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat Nanotechnol. 2021;16:243–50.
Cunningham FJ, Demirer GS, Goh NS, Zhang H, Landry MP. Nanobiolistics: an emerging genetic transformation approach. Berlin: Springer; 2020.
Jiang M, Song Y, Kanwar MK, Ahammed GJ, Shao S, Zhou J. Phytonanotechnology applications in modern agriculture. J Nanobiotechnol. 2021;19:1–20.
Zhang Q, Ying Y, Ping J. Recent advances in plant nanoscience. Adv Sci. 2022;9:2103414.
Cunningham FJ, Goh NS, Demirer GS, Matos JL, Landry MP. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018;36:882–97.
Kandhol N, Singh VP, Herrera-Estrella L, Tran L-SP, Tripathi DK. Nanocarrier spray: a nontransgenic approach for crop engineering. Trends Plant Sci. 2023;28:259–61.
Naqvi S, Maitra A, Abdin M, Akmal M, Arora I, Samim M. Calcium phosphate nanoparticle mediated genetic transformation in plants. J Mater Chem. 2012;22:3500–7.
Mendes BB, Conniot J, Avital A, Yao D, Jiang X, Zhou X, Sharf-Pauker N, Xiao Y, Adir O, Liang H. Nanodelivery of nucleic acids. Nat Rev Methods Primers. 2022;2:24.
Hao Y, Yang X, Shi Y, Song S, Xing J, Marowitch J, Chen J, Chen J. Magnetic gold nanoparticles as a vehicle for fluorescein isothiocyanate and DNA delivery into plant cells. Botany. 2013;91:457–66.
George, A.C.S. Magnetofection of tobacco protoplasts: a novel mechanism for plant transformation. Murdoch University, 2018
Zhao X, Meng Z, Wang Y, Chen W, Sun C, Cui B, Cui J, Yu M, Zeng Z, Guo S. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat plants. 2017;3:956–64.
Wang ZP, Zhang ZB, Zheng DY, Zhang TT, Li XL, Zhang C, Yu R, Wei JH, Wu ZY. Efficient and genotype independent maize transformation using pollen transfected by DNA-coated magnetic nanoparticles. J Integr Plant Biol. 2022;64:1145–56.
Jia YJ, Guo X, Cheng FY. Preliminary analysis on transgenic technology of pollen-mediated by magnetic nanoparticles in tree peony. Mol Plant Breed. 2020;18:2577–84 ((in Chinese)).
Cao SS, Xue J, Chen XQ, Wang QN, Zhang XH, An XH. Establishment of Chlorella ellipsoidea transformation system mediated by magnetic nanoparticles. Jiangsu J Agric Sci. 2020;36:306–11 ((in Chinese)).
Sun GS, Yang Y, Xu KC, Zhang CW, Dai ZL, Sun CQ, Ma ZH. Establishment of magnetic nanoparticles-mediated genetic transformation aystem in Capsicum annuum L. J Nucle Agric Sci. 2021;35:1307–12 ((in Chinese)).
Wang P, Lombi E, Zhao F-J, Kopittke PM. Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 2016;21:699–712.
Peng Z, Liu X, Zhang W, Zeng Z, Liu Z, Zhang C, Liu Y, Shao B, Liang Q, Tang W. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: a review. Environ Int. 2020;134:105298.
Li Y, Xu X, Wu Y, Zhuang J, Zhang X, Zhang H, Lei B, Hu C, Liu Y. A review on the effects of carbon dots in plant systems. Mater Chem Front. 2020;4:437–48.
Liu Q, Chen B, Wang Q, Shi X, Xiao Z, Lin J, Fang X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009;9:1007–10.
Burlaka O, Pirko YV, Yemets A, Blume YB. Plant genetic transformation using carbon nanotubes for DNA delivery. Cytol Genet. 2015;49:349–57.
Golestanipour A, Nikkhah M, Aalami A, Hosseinkhani S. Gene delivery to tobacco root cells with single-walled carbon nanotubes and cell-penetrating fusogenic peptides. Mol Biotechnol. 2018;60:863–78.
Demirer GS, Zhang H, Matos JL, Goh NS, Cunningham FJ, Sung Y, Chang R, Aditham AJ, Chio L, Cho M-J. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat Nanotechnol. 2019;14:456–64.
Kwak S-Y, Lew TTS, Sweeney CJ, Koman VB, Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua N-H, Strano MS. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat Nanotechnol. 2019;14:447–55.
Lew TTS, Park M, Wang Y, Gordiichuk P, Yeap W-C, Mohd Rais SK, Kulaveerasingam H, Strano MS. Nanocarriers for transgene expression in pollen as a plant biotechnology tool. ACS Mater Lett. 2020;2:1057–66.
Ghaghelestany AB, Jahanbakhshi A, Taghinezhad E. Gene transfer to German chamomile (L chamomilla M) using cationic carbon nanotubes. Sci Hortic. 2020;263:109106.
Wang B, Huang J, Zhang M, Wang Y, Wang H, Ma Y, Zhao X, Wang X, Liu C, Huang H. Carbon dots enable efficient delivery of functional DNA in plants. ACS Appl Bio Mater. 2020;3:8857–64.
Doyle C, Higginbottom K, Swift TA, Winfield M, Bellas C, Benito-Alifonso D, Fletcher T, Galan MC, Edwards K, Whitney HM. A simple method for spray-on gene editing in planta. BioRxiv. 2019. https://doi.org/10.1101/805036v2.abstract.
Ali S, Dussouillez C, Padilla B, Frisch B, Mason AJ, Kichler A. Design of a new cell penetrating peptide for DNA, siRNA and mRNA delivery. J Gene Med. 2022;24:e3401.
Unnamalai N, Kang BG, Lee WS. Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells. FEBS Lett. 2004;566:307–10.
Rosenbluh J, Singh SK, Gafni Y, Graessmann A, Loyter A. Non-endocytic penetration of core histones into petunia protoplasts and cultured cells: a novel mechanism for the introduction of macromolecules into plant cells. Biochim Biophys Acta (BBA) Biomembr. 2004;1664:230–40.
Lu S-W, Hu J-W, Liu BR, Lee C-Y, Li J-F, Chou J-C, Lee H-J. Arginine-rich intracellular delivery peptides synchronously deliver covalently and noncovalently linked proteins into plant cells. J Agric Food Chem. 2010;58:2288–94.
Chugh A, Eudes F. Study of uptake of cell penetrating peptides and their cargoes in permeabilized wheat immature embryos. FEBS J. 2008;275:2403–14.
Lakshmanan M, Kodama Y, Yoshizumi T, Sudesh K, Numata K. Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromol. 2013;14:10–6.
Chuah J-A, Yoshizumi T, Kodama Y, Numata K. Gene introduction into the mitochondria of Arabidopsis thaliana via peptide-based carriers. Sci Rep. 2015;5:7751.
Yoshizumi T, Oikawa K, Chuah J-A, Kodama Y, Numata K. Selective gene delivery for integrating exogenous DNA into plastid and mitochondrial genomes using peptide–DNA complexes. Biomacromol. 2018;19:1582–91.
Thagun C, Chuah JA, Numata K. Targeted gene delivery into various plastids mediated by clustered cell-penetrating and chloroplast-targeting peptides. Adv Sci. 2019;6:1902064.
Torney F, Trewyn BG, Lin VS-Y, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol. 2007;2:295–300.
Martin-Ortigosa S, Valenstein JS, Lin VSY, Trewyn BG, Wang K. Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv Func Mater. 2012;22:3576–82.
Martin-Ortigosa S, Peterson DJ, Valenstein JS, Lin VS-Y, Trewyn BG, Lyznik LA, Wang K. Mesoporous silica nanoparticle-mediated intracellular Cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014;164:537–47.
Chang F-P, Kuang L-Y, Huang C-A, Jane W-N, Hung Y, Yue-ie CH, Mou C-Y. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J Mater Chem B. 2013;1:5279–87.
Hajiahmadi Z, Shirzadian-Khorramabad R, Kazemzad M, Sohani MM. Enhancement of tomato resistance to Tuta absoluta using a new efficient mesoporous silica nanoparticle-mediated plant transient gene expression approach. Sci Hortic. 2019;243:367–75.
Vejlupkova Z, Warman C, Sharma R, Scheller HV, Mortimer JC, Fowler JE. No evidence for transient transformation via pollen magnetofection in several monocot species. Nat plants. 2020;6:1323–4.
Jat SK, Bhattacharya J, Sharma MK. Nanomaterial based gene delivery: a promising method for plant genome engineering. J Mater Chem B. 2020;8:4165–75.
Zhang J, Yu D, Zhang Y, Liu K, Xu K, Zhang F, Wang J, Tan G, Nie X, Ji Q. Vacuum and co-cultivation agroinfiltration of (germinated) seeds results in tobacco rattle virus (TRV) mediated whole-plant virus-induced gene silencing (VIGS) in wheat and maize. Front Plant Sci. 2017;8:393.
Demirer GS, Landry MP. Efficient transient gene knock-down in tobacco plants using carbon nanocarriers. Bio-Protoc. 2021;11:e3897–e3897.
Schwartz SH, Hendrix B, Hoffer P, Sanders RA, Zheng W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020;184:647–57.
Li S, Li J, Du M, Deng G, Song Z, Han H. Efficient gene silencing in intact plant cells using siRNA delivered by functional graphene oxide nanoparticles. Angew Chem. 2022;134:e202210014.
Cai Y, Liu Z, Wang H, Meng H, Cao Y. Mesoporous silica nanoparticles mediate SiRNA delivery for long-term multi-gene silencing in intact plants. Adv Sci. 2024;11:2301358.
Liu Z, Zhang J, Cai Y, Wang H, Luo M, Li J, Yu H, Meng X, Cao Y. Improving seed shattering resistance in Wild O. alta rice with mesoporous silica nanoparticle delivery systems. Nano Lett. 2024;24:11823–30.
Lei W-X, An Z-S, Zhang B-H, Wu Q, Gong W-J, Li J-M, Chen W-L. Construction of gold-siRNA NPR1 nanoparticles for effective and quick silencing of NPR1 in Arabidopsis thaliana. RSC Adv. 2020;10:19300–8.
Zhang H, Cao Y, Xu D, Goh NS, Demirer GS, Cestellos-Blanco S, Chen Y, Landry MP, Yang P. Gold-nanocluster-mediated delivery of siRNA to intact plant cells for efficient gene knockdown. Nano Lett. 2021;21:5859–66.
Zhang H, Goh NS, Wang JW, Pinals RL, González-Grandío E, Demirer GS, Butrus S, Fakra SC, Del Rio Flores A, Zhai R. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat Nanotechnol. 2022;17:197–205.
Liu Q, Li Y, Xu K, Li D, Hu H, Zhou F, Song P, Yu Y, Wei Q, Liu Q. Clay nanosheet-mediated delivery of recombinant plasmids expressing artificial miRNAs via leaf spray to prevent infection by plant DNA viruses. Hortic Res. 2020;7:179.
Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu G, Xu ZP. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat plants. 2017;3:1–10.
Yong J, Zhang R, Bi S, Li P, Sun L, Mitter N, Carroll BJ, Xu ZP. Sheet-like clay nanoparticles deliver RNA into developing pollen to efficiently silence a target gene. Plant Physiol. 2021;187:886–99.
Zhang H, Demirer GS, Zhang H, Ye T, Goh NS, Aditham AJ, Cunningham FJ, Fan C, Landry MP. DNA nanostructures coordinate gene silencing in mature plants. Proc Natl Acad Sci. 2019;116:7543–8.
Miyamoto T, Tsuchiya K, Numata K. Dual peptide-based gene delivery system for the efficient transfection of plant callus cells. Biomacromol. 2020;21:2735–44.
Thagun C, Horii Y, Mori M, Fujita S, Ohtani M, Tsuchiya K, Kodama Y, Odahara M, Numata K. Non-transgenic gene modulation via spray delivery of nucleic acid/peptide complexes into plant nuclei and chloroplasts. ACS Nano. 2022;16:3506–21.
Demirer GS, Zhang H, Goh NS, Pinals RL, Chang R, Landry MP. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci Adv. 2020;6:eaaz0495.
Francis AP, Devasena T. Toxicity of carbon nanotubes: a review. Toxicol Ind Health. 2018;34:200–10.
Chen J, Dou R, Yang Z, Wang X, Mao C, Gao X, Wang L. The effect and fate of water-soluble carbon nanodots in maize (Zea mays L.). Nanotoxicology. 2016;10:818–28.
Chen J, Liu B, Yang Z, Qu J, Xun H, Dou R, Gao X, Wang L. Phenotypic, transcriptional, physiological and metabolic responses to carbon nanodot exposure in Arabidopsis thaliana (L.). Environ Sci: Nano. 2018;5:2672–85.
Ali Z, Serag M, Demirer G, Torre B, di Fabrizio E, Landry M, Habuchi S, Mahfouz M. The DNA–carbon nanotube binding mode determines the efficiency of carbon nanotube-mediated DNA delivery to intact plants. ACS Appl Nano Mater. 2021;5(4):4663–76.
Lv Z, Jiang R, Chen J, Chen W. Nanoparticle-mediated gene transformation strategies for plant genetic engineering. Plant J. 2020;104:880–91.
Demirer GS, Zhang H, Goh NS, González-Grandío E, Landry MP. Carbon nanotube–mediated DNA delivery without transgene integration in intact plants. Nat Protoc. 2019;14:2954–71.
Wang JW, Grandio EG, Newkirk GM, Demirer GS, Butrus S, Giraldo JP, Landry MP. Nanoparticle-mediated genetic engineering of plants. Mol Plant. 2019;12:1037–40.
Avellan A, Yun J, Zhang Y, Spielman-Sun E, Unrine JM, Thieme J, Li J, Lombi E, Bland G, Lowry GV. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano. 2019;13:5291–305.
Ma C, White JC, Zhao J, Zhao Q, Xing B. Uptake of engineered nanoparticles by food crops: characterization, mechanisms, and implications. Annu Rev Food Sci Technol. 2018;9:129–53.
Wong MH, Misra RP, Giraldo JP, Kwak S-Y, Son Y, Landry MP, Swan JW, Blankschtein D, Strano MS. Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: a universal localization mechanism. Nano Lett. 2016;16:1161–72.
Lv J, Christie P, Zhang S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ Sci Nano. 2019;6:41–59.
Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, Wiesner MR. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology. 2016;10:257–78.
Wang ZW, Gao X, Ma DJ, Zhong S, Liu XL, Xi Z. Nucleic acid pesticides-the new plant protection products with great potential. Chin J Pest Sci. 2019;21:681–91 ((in Chinese)).
Koo Y, Wang J, Zhang Q, Zhu H, Chehab EW, Colvin VL, Alvarez PJ, Braam J. Fluorescence reports intact quantum dot uptake into roots and translocation to leaves of Arabidopsis thaliana and subsequent ingestion by insect herbivores. Environ Sci Technol. 2015;49:626–32.
Cao J, Feng Y, Lin X, Wang J, Xie X. Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant. J Soils Sediments. 2017;17:841–51.
Pan LS, Wang WC, Yao MY, Wang XY, Zhang XZ. Research progress on plant-derived exosome-like nanoparticles and their applications. China J Chin Materia Med. 2023;48:5977–84 ((in Chinese)).
Hillman T. The use of plant-derived exosome-like nanoparticles as a delivery system of CRISPR/Cas9-based therapeutics for editing long non-coding RNAs in cancer colon cells. Front Oncol. 2023;13:1194350.
Bost JP, Barriga H, Holme MN, Gallud A, Maugeri M, Gupta D, Lehto T, Valadi H, Esbjorner EK, Stevens MM. Delivery of oligonucleotide therapeutics: chemical modifications, lipid nanoparticles, and extracellular vesicles. ACS Nano. 2021;15:13993–4021.
Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release. 2017;266:8–16.
Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5:1700611.
McAndrews KM, Xiao F, Chronopoulos A, LeBleu VS, Kugeratski FG, Kalluri R. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Sci Alliance. 2021. https://doi.org/10.26508/lsa.202000875.
Majeau N, Fortin-Archambault A, Gérard C, Rousseau J, Yaméogo P, Tremblay JP. Serum extracellular vesicles for delivery of CRISPR-CAS9 ribonucleoproteins to modify the dystrophin gene. Mol Ther. 2022;30:2429–42.
Mun D, Kang J-Y, Kim H, Yun N, Joung B. Small extracellular vesicle-mediated CRISPR-Cas9 RNP delivery for cardiac-specific genome editing. J Control Release. 2024;370:798–810.
Wan Y, Li L, Chen R, Han J, Lei Q, Chen Z, Tang X, Wu W, Liu S, Yao X. Engineered extracellular vesicles efficiently deliver CRISPR-Cas9 ribonucleoprotein (RNP) to inhibit herpes simplex virus1 infection in vitro and in vivo. Acta Pharm Sin B. 2024;14:1362–79.
Hamby R, Cai Q, Jin H. RNA communication between organisms inspires innovative eco-friendly strategies for disease control. Nat Rev Mol Cell Biol. 2024;26:1–2.
Cai Q, Halilovic L, Shi T, Chen A, He B, Wu H, Jin H. Extracellular vesicles: cross-organismal RNA trafficking in plants, microbes, and mammalian cells. Extracell Vesicles Circulat Nucle Acids. 2023;4:262.
He B, Wang H, Liu G, Chen A, Calvo A, Cai Q, Jin H. Fungal small RNAs ride in extracellular vesicles to enter plant cells through clathrin-mediated endocytosis. Nat Commun. 2023;14:4383.
Cai Q, Qiao L, Wang M, He B, Lin F-M, Palmquist J, Huang S-D, Jin H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–9.
Wang S, He B, Wu H, Cai Q, Ramírez-Sánchez O, Abreu-Goodger C, Birch PR, Jin H. Plant mRNAs move into a fungal pathogen via extracellular vesicles to reduce infection. Cell Host Microbe. 2024;32(93–105):e106.
Yin C, Lao Y, Xie L, Chen L, Jiang Y, Gong L. Citrus exosome-modified exogenous dsRNA delivery reduces plant pathogen resistance and mycotoxin production. Pestic Biochem Physiol. 2024;205:106151.
Landry MP, Mitter N. How nanocarriers delivering cargos in plants can change the GMO landscape. Nat Nanotechnol. 2019;14:512–4.
Raza A, Charagh S, Salehi H, Abbas S, Saeed F, Poinern GE, Siddique KH, Varshney RK. Nano-enabled stress-smart agriculture: can nanotechnology deliver drought and salinity-smart crops? J Sustain Agric Environ. 2023;2:189–214.
Torti S, Schlesier R, Thümmler A, Bartels D, Römer P, Koch B, Werner S, Panwar V, Kanyuka K, Wirén NV. Transient reprogramming of crop plants for agronomic performance. Nat Plants. 2021;7:159–71.
Funding
This work was supported by the Key-Area Research and Development Program of Guangdong Province (NO.2020B020215003), Forestry Science and Technology Innovation Project of Guangdong Province (2024KJCX004), Scarce and Quality Economic Forest Engineering Technology Research Center (NO.2022GCZX002), Guangzhou Science and Technology Project (NO.2024E04J0067), and Heyuan Science and Technology Project (NO.240201111470644).
Author information
Authors and Affiliations
Contributions
SL, YZ: data analysis, visualization, drafted the manuscript. LP, WW, YL: inputs and revision. ZL, XZ: conceptualization, funding acquisition, revised the manuscript. All authors read and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Zheng, Y., Pan, L. et al. Nanodelivery of nucleic acids for plant genetic engineering. Discover Nano 20, 31 (2025). https://doi.org/10.1186/s11671-025-04207-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-025-04207-9