- Research
- Open access
- Published:
Production of minicell-like structures by Escherichia coli biosynthesizing cadmium fluorescent nanoparticles: a novel response to heavy metal exposure
Journal of Nanobiotechnology volume 23, Article number: 111 (2025)
Abstract
The bacterial synthesis of fluorescent semiconductor nanoparticles or quantum dots (QDs), presents a sustainable method for producing nanomaterials with customized optical properties and significant technological potential. However, the underlying cellular mechanisms for this process remain elusive. Specifically, the role of cellular structures in QD generation has not been thoroughly investigated. In this study, we examined the morphological changes in Escherichia coli during the biosynthesis of cadmium sulfide (CdS) QDs, using a strain overexpressing the gshA gene to promote QD biosynthesis through increased glutathione (GSH) levels. Microscopy analyses revealed that fluorescence emission associated with QDs was concentrated at the cell poles, along with fluorescence emission from small spherical cells, a phenomenon exclusively detectable during QD biosynthesis. Transmission electron microscopy (TEM) revealed electron-dense nanomaterials localized at the cell poles. Furthermore, it was demonstrated the formation of minicell-like structures (∼ 0.5 μm in diameter) originating from these poles under biosynthesis conditions. These minicells encapsulated nanometric electron-dense material. Additional analyses indicated that minicells contained inclusion bodies, likely formed due to gshA overexpression and cadmium stress. Our findings confirms the role of minicells as a bacterial mechanism for sequestering cadmium at the cell poles and expelling the metal in the form of nanoparticles. This underscores the importance of minicells in bacterial physiology and stress responses, introducing a novel mechanism for heavy metal detoxification in bacteria.
Introduction
The study of bacterial detoxification mechanisms as a means of achieving metal resistance has been crucial for understanding cellular survival and adaptation to the various stresses caused by metal exposure [1]. One of the ways bacterial cells have adapted to cope with heavy metal exposure is through the biosynthesis of quantum dot nanoparticles, a mechanism that immobilizes harmful metal ions [2]. Quantum dots (QDs) are semiconductor fluorescent nanoparticles characterized by a size of 1 to 10 nm in all their dimensions, and are composed of elements such as cadmium, copper, zinc, sulfur, or tellurium, among others [3, 4]. The fluorescence emission of QDs arises from the quantum confinement effect [5]. This unique effect enables the precise control of their optical properties by manipulating the chemical composition and size of the nanoparticles [6]. Thanks to these tunable properties, QDs have garnered extensive attention in a variety of applications, including their use as sensitizers in solar cells [7], organic pollutant degradation [8], and hydrogen production [9], among others. The significance of these nanoparticles was further recognized in 2023 when the Nobel Prize in Chemistry was awarded to Aleksey I. Ekimov, Louis E. Brus, and Moungi G. Bawendi for their pioneering work in the discovery and synthesis of QDs [10].
To date, numerous methods for the biological synthesis of QDs have been reported. Biosynthesis strategies leverage cells or cell-derived biomolecules that interact with metal precursors to facilitate the generation of nanomaterials [11]. Among the various systems employed for QDs biosynthesis, bacterial cells stand out as an attractive platform due to their simple culture conditions and rapid growth [12]. Moreover, QDs synthesized by bacteria exhibit an organic coating of proteins and other biomolecules which mitigate their toxicity and contribute to nanoparticle stabilization [13, 14].
Numerous studies have investigated the factors involved in the bacterial synthesis of QDs, employing cadmium-based nanoparticles as a model. In Escherichia coli, cadmium is assimilated into the cell through the phosphate inorganic transport system (Pit) as a metal-phosphate complex [15, 16]. Consequently, phosphate concentration has been identified as a crucial factor promoting QDs biosynthesis [17]. Other elements involved in the biosynthesis of cadmium QDs are molecules derived from sulfur metabolism. Cysteine and the production of hydrogen sulfide (H2S), for instance, have been extensively studied as catalysts for the biosynthesis of cadmium sulfide (CdS) QDs [18,19,20,21]. The significance of glutathione (GSH) in the biosynthesis of CdS and cadmium telluride (CdTe) QDs has also been reported [14], underscoring the role of antioxidant defenses in QDs biosynthesis. Additionally, different proteins interacting with cadmium ions and contributing to the formation and stabilization of the nanostructure have been proposed as a third element in the biosynthesis process [13, 22,23,24]. However, there are still few studies that have examined the impact of QD biosynthesis on cell morphology.
In a previous study, our research group reported the biosynthesis of cadmium QDs utilizing a genetically modified strain of E. coli [14]. Specifically, the strain AG1/pCA24NgshA overexpressesing the gshA gene, encoding the L-glutamate-cysteine ligase enzyme [25], was employed for the synthesis of QDs. The overexpression of this gene resulted in a two-fold increase in the total cellular content of GSH, enabling the intracellular biosynthesis of CdS QDs following exposure to CdCl2 [14]. Notably, in this system, fluorescence emission was localized in specific cell structures, concentrated at cell poles or in the extracellular environment. This intriguing observation suggests that the bacterial QDs biosynthesis could be related to cell membrane stress or triggers a non-canonical division process. Thus, we propose that in the system of E. coli AG1/pCA24NgshA, the biosynthesis process involves the formation of vesicles or minicell-like structures. Minicells are spherical cell structures generated through polar cell division, which lack chromosomal DNA and consequently are incapable of further division [26]. These structures emerge in strains with mutations in the Min division system [27,28,29], responsible for ensuring proper division at the cell center [30].
Notably, various studies have examined the physiological role of minicells in detoxifying cells from different toxic components. For example, the formation of minicells enhances the cell’s tolerance to streptomycin by expelling misfolded proteins and inclusion bodies [31]. Additionally, strains that produce minicells have been shown to exhibit increased tolerance to various toxic chemicals, such as isobutyraldehyde, isobutanol, and isobutyl acetate [32]. In a recent study, our research group investigated the impact of the minicell-producing phenotype on the biosynthesis of CdS nanoparticles and demonstrated that minicells serve as a platform for accumulating and expelling nanoparticles from the cell [33]. These findings support the hypothesis that minicells represent a general mechanism for stress tolerance in E. coli, which extends to QDs biosynthesis. However, whether the minicell phenotype can be induced in wild-type cells under nanoparticle biosynthesis conditions remains unknown.
Leveraging these precedents, we conducted a morphological study of the E. coli AG1/pCA24NgshA system during biosynthesis of CdS QDs. Our investigation involved the assessment of fluorescence emission patterns and, notably, the identification of minicell-like structures, establishing a novel association between minicell formation and QD biosynthesis for the encapsulation and disposal of fluorescent nanoparticles and thus of heavy metals.
Materials and methods
Bacterial strains
E. coli strains AG1/pCA24N (wild type, empty vector) and AG1/pCA24NgshA (over expressing the E. coli gshA gene) were obtained from the ASKA collection [34]. Strains were routinely grown at 37 °C in lysogeny broth (LB; composition: 0.5% yeast extract, 1% tryptone, 1% NaCl) supplemented with 25 µg/ml chloramphenicol. Strains were kept in LB plates (2% agar) supplemented with chloramphenicol at 4 °C.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE)
Total protein analysis was performed by standard SDS-PAGE [35]. Bacterial pellets obtained after different conditions were disrupted by sonication. Samples were centrifuged at 14,000 x g for 10 min and the supernatant was recovered as the soluble protein fraction. The pellet was resuspended in urea 8 M and mixed thoroughly at room temperature to obtain the insoluble protein fraction [36]. Protein concentration was quantified by the Bradford method, using the Bio-Rad Protein Assay Dye Reagent (Bio-Rad Laboratories). Samples of 10 µg of protein were fractionated in 12% polyacrylamide gels. “Blue Prestained Protein Standard, Broad Range (11–250 kDa)” (New England Biolabs) was used as molecular weight marker. Proteins were visualized by staining with Coomassie brilliant blue R-250 [37].
Biosynthesis of CdS nanoparticles
Biosynthesis of nanoparticles was performed as described by Monrás et al. (2012) [14]. A single colony of E. coli AG1/pCA24NgshA was grown overnight in LB broth supplemented with 25 µg/ml of chloramphenicol at 37 °C with constant agitation. This culture was diluted in a ratio of 1:50 in fresh broth and then incubated at 37 °C with constant agitation until OD600 ∼ 0.5 was reached. Then, 0.5 mM of Isopropyl-b-D-1-thiogalactopyranoside (IPTG; Thermo Scientific) was added to the culture and incubated for 4 h. After induction with IPTG, cells were washed with LB broth, resuspended in an equal volume of broth and exposed to 10 µg/ml of CdCl2. Subsequently, the cells were incubated at 37 °C for 18 h. Cells were harvested by centrifugation (7690 x g for 30 min) and washed with 50 mM potassium phosphate buffer, pH 7.4. Cell pellets were used for further characterization. Control experiments were performed under the same conditions without the addition of CdCl2 or using the E. coli strain AG1/pCA24N, carrying the empty vector.
Nanoparticle purification
Nanoparticle preparation from cell pellets was conducted as previously reported [33]. Cell pellets obtained from 100 ml of biosynthesis cultures were suspended in 10 ml of a 1 M NaOH solution and then incubated at 90 °C for 10 min. Pellets were recovered by centrifugation and then resuspended in 10 ml of 50 mM Tris-HCl buffer, pH 8.5 with 1% SDS and disrupted by sonication. The cell debris was removed by centrifugation and filtration with a 0.22 μm filter. Nanoparticles were concentrated in 10 kDa Amicon tubes (Millipore) to an approximate volume of 200 µl. The optical properties of these solutions were analyzed in a Synergy H1 microplate reader (BioTek Instrument Inc.). Absorbance spectra were recorded between 300 and 700 nm. Fluorescence spectra were measured between 470 and 700 nm, with excitation at 360 nm.
Fluorescence microscopy
Cell pellets obtained from 10 ml of biosynthesis cultures were washed and resuspended in 200 µl of 50 mM potassium phosphate buffer, pH 7.4 and a 5 µl aliquot was mounted on a glass slide without fixation for observation on a MF606 epifluorescence microscope (BW OPTICS). Samples were analyzed with a 40X objective (NA: 0.65). Fluorescence images were captured after excitation with a 330–380 nm filter. Fluorescence intensity profiles were obtained using the Fiji-ImageJ program, by manually drawing a line along the long axis of cells of fluorescence images. Profiles were graphed using the R software, and a tendency line was generated using the geom_smooth function from the “tidyverse” library. For length dynamics analysis, three random fields of view were selected for each condition from two biological replicates, and cell-length was measured manually using the Fiji-ImageJ program.
Transmission electron microscopy (TEM)
Cell pellets obtained from 10 ml of biosynthesis cultures were fixed with glutaraldehyde 2.5%, stained with osmium tetroxide 1% and infiltrated with epoxy resin. Ultrathin sections were obtained using an ultramicrotome and placed in commercial copper grids. Micrographs were collected using a Talos F200C G2 microscope (Thermo Fisher Scientific), operated at 200 kV.
Statistical analysis
All statistical analyses were conducted using R software. Group comparisons were performed using the Kruskal-Wallis test with the compare_means function. Post-hoc analysis was conducted using Dunn’s test, implemented through the dunnTest function from the “dunn.test” and “FSA” libraries. A p value < 0.05 was considered as significant. Clustering analysis of treated cells was carried out using k-means clustering with k = 12, dividing each group into three clusters. The Gower distance was used to account for both continuous numerical and categorical variables [38].
Results
CdS nanoparticle biosynthesis affects the size dynamics of E. coli and relates to the formation of small cells
Previously, our group reported the intracellular biosynthesis of CdS QDs in the E. coli AG1/pCA24NgshA strain. Nanoparticle biosynthesis was promoted by over-expression of the gshA gene and subsequent increase in total GSH content [14]. In this work, we used the same strategy to stimulate biosynthesis of QDs and to study its physiological effects in E. coli. We first confirmed the expression of the GshA protein in the AG1/pCA24NgshA strain. Total protein analysis through SDS-PAGE of strain AG1/pCA24NgshA corroborates the expression of GshA protein after induction with IPTG for 4 h, evaluated by the increase of intensity of a band of ∼ 58 kDa (Figure S1), concordant with the weight of GshA protein [39].
After induction, cells were exposed to CdCl2 for CdS nanoparticle biosynthesis. Because of their intrinsic fluorescence emission, QDs biosynthesis was monitored by exposing cell pellets to UV light (365 nm). As previously reported [14], IPTG-induced cells exposed to CdCl2 exhibited fluorescence emission, indicative of the intracellular biosynthesis of cadmium nanoparticles (Fig. 1A). Fluorescence emission was not visible in the strain AG1/pCA24N, carrying the empty vector, corroborating the need for the increased GSH content for QDs biosynthesis in this system [14].
Intracellular biosynthesis of CdS nanoparticles. (A) Bacterial pellets of the indicated E. coli strains after biosynthesis conditions with exposure to CdCl2 or without metal treatment (control) after exposure to UV light (365 nm). (B) Absorption and fluorescence emission spectra of purified nanoparticles. Absorption spectrum was recorded between 300 and 700 nm. Fluorescence emission spectrum was recorded between 470 and 700 nm, with 360 nm excitation. (C) Fluorescence microscopy images of E. coli AG1/pCA24NgshA cells from (A). Fluorescence images were obtained after excitation with a 330–380 nm filter. Scale bar = 10 μm. (D) Fluorescence intensity profile of AG1/pCA24NgshA cells after biosynthesis of CdS QDs, along the normalized long cell axis. Each line is a profile of a single cell (n = 120). Bold black line represents the tendency line of the profiles
To confirm if fluorescence emission is a consequence of the biosynthesis of cadmium QDs, these were recovered from cell pellets and their optical properties were analyzed. Purified QDs showed an absorption peak below 400 nm, while a broad emission peak was observed between 500 and 550 nm (Fig. 1B). These optical properties are characteristic of cadmium-based QDs [14, 17, 40, 41], which indicates that cadmium QDs were biosynthesized.
To elucidate if fluorescence emission comes from the inside of the cell, cell pellets were analyzed by fluorescence microscopy. Cells treated with CdCl2 showed fluorescence emission from individual cells (Fig. 1C). In contrast, cells of strain AG1/pCA24N showed no fluorescence emission in any condition (Figure S2). These results confirm that intracellular QDs are being synthesized in strain AG1/pCA24NgshA.
In our previous report, we observed that fluorescence intensity is primarily concentrated on structures at the cell poles [14]. In the microscopy analysis conducted in this study, we confirmed high fluorescence at the cell poles (Fig. 1C). Measurements of fluorescence intensity along the long axis of QD-containing cells revealed that most cells exhibited a peak in intensity at one or both poles (Fig. 1D), supporting this observation.
In a significant proportion of cells, fluorescence emission was concentrated at either one (Fig. 2A and B) or both cellular poles (Fig. 2 C, 2D), representing approximately 27% and 53% of the population, respectively. This emission pattern suggests that fluorescent nanoparticles are either synthesized directly at the cell poles or relocated there after synthesis. The latter seems more plausible, given the presence of fluorescence emission throughout the entire cell. Additionally, we observed cells with a random distribution of fluorescence emission (Fig. 2F and G), accounting for approximately 20% of the population. This pattern indicates that the initiation sites for nanoparticle synthesis are dispersed throughout the cell. Upon closer examination of the fluorescent cells producing QDs, we detected fluorescence emission from small, spherical cells that appeared to be separated from rod-shaped cells (Fig. 2H and I). This observation suggests a link between nanoparticle biosynthesis and a process of cell fragmentation. If these spherical cells indeed originate from rod-shaped cells, the emission from cell poles could represent an intermediate stage before the release of the polar structure, like in minicell production [31]. An intriguing observation was that regions of higher fluorescence emission coincided with denser zones in the cell, as observed through optical microscopy (Fig. 2). This correlation suggests that the increased fluorescence emission is due to the accumulation of cellular elements and cadmium metal.
Fluorescence emission patterns of E. coli AG1/pCA24NgshA cells after exposure to CdCl2. Images show representative regions of interest displaying fluorescence emission from cell poles (A, B, C, D), random distribution (F, G) or spherical cells (H, I). Red arrows indicate zones of higher fluorescence emission. Fluorescence images were captured after excitation with a 330–380 nm filter
The formation of spherical cells due to CdS QD biosynthesis could potentially impact the overall cell population size. To assess the effect of nanoparticle formation on cell size distribution, we analyzed the dynamics of cell length under biosynthesis conditions, visualized using violin plots (Fig. 3).
Cell-length distribution after biosynthesis conditions. (A) Violin plots of cell length of the indicated E. coli strains after exposure to QDs biosynthesis conditions, with metal added (CdCl2) or without metal added (control). Each dot represents an individual cell. Comparison between groups was analyzed with a Kruskal-Wallis test (p = 3.9e− 81). Cell number: AG1/pCA24N control (n = 822), AG1/pCA24N CdCl2 (n = 872), AG1/pCA24NgshA control (n = 1055), AG1/pCA24NgshA CdCl2 (n = 1012). (B) Violin plots of the smallest cell clusters from each group in (A), depicting cell length of the indicated E. coli strains after exposure to QDs biosynthesis conditions, with metal added (CdCl2) or without metal added (control). Box plots indicate the 25%-quartile, median and 75%-quartile. Comparison between groups was analyzed with a Kruskal-Wallis test (p = 3.3e− 138). Cell number: AG1/pCA24N control (n = 327), AG1/pCA24N CdCl2 (n = 369), AG1/pCA24NgshA control (n = 451), AG1/pCA24NgshA CdCl2 (n = 175)
When comparing the size distribution of the AG1/pCA24NgshA strain to the control strain, we consistently observed smaller cell sizes, regardless of metal exposure. This finding suggests that overexpression of the GshA protein has a significant impact on overall cell size. Additionally, cadmium exposure also contributes to differences between groups, although in the AG1/pCA24N strain, the addition of cadmium results in only a minor change in size. Interestingly, although both strains, whether under control conditions or exposed to CdCl2, exhibited one predominant cell size, the AG1/pCA24NgshA strain under CdCl2 exposure displayed a secondary cell population with sizes fluctuating around 1 μm, a characteristic size of minicells [42, 43]. This observation is noteworthy because minicell sizes are not uniform [44], indicating that this secondary population may consist of minicell-like structures of varying sizes.
To evaluate the differences in cell subpopulations due to different treatments, we measured the Gower distance between cells from the different groups shown in Fig. 3A and generated three subgroups for each (Figure S3). Cluster analysis revealed that both cadmium exposure and overexpression of the GshA protein are associated with a tendency towards smaller cell sizes within subgroups (Figure S3). This trend is particularly evident when examining the smallest cell clusters in each group (Fig. 3B). In the AG1/pCA24N strain, cadmium addition is linked to a slight reduction in cell size, suggesting that cadmium exposure contributes to decreased cell size in the population. This effect is further amplified in the AG1/pCA24NgshA strain, indicating that GshA overexpression is also related to reduced cell size. Finally, the combination of GshA protein overexpression and cadmium exposure, which leads to QD biosynthesis, has a synergistic effect on cell size, suggesting that CdS QD biosynthesis is closely associated with the generation of small cells and may explain the appearance of the small spherical cells observed (Fig. 2H and I).
Expression of GshA protein involves inclusion body formation
To further investigate the morphological changes in E. coli and the localization of nanoparticles within the cells, we conducted an analysis using TEM. After exposing the control strain AG1/pCA24N to CdCl2, the cells appeared like those under control conditions (Fig. 4A and B), consistent with our previous observation of only slight changes due to the low cadmium concentration used (Fig. 3). In contrast, cells from the AG1/pCA24NgshA strain under both conditions displayed well-defined oval electron-dense structures (Fig. 4 C, 4D, 4E, 4 F). Given that these cells are overexpressing the gshA gene (Figure S1), these structures could be inclusion bodies formed by the aggregation of the GshA protein. This is further supported by the determination of the proportion of cells containing electron-dense material, which indicated that, in the absence of CdCl2, the AG1/pCA24NgshA strain has a higher proportion of cells displaying electron-dense material than the control AG1/pCA24N strain, a proportion that increases further under metal exposure in both strains (Fig. 4G). Moreover, SDS-PAGE analysis of insoluble proteins from the AG1/pCA24NgshA strain after IPTG induction revealed that part of the GshA protein aggregates, a phenomenon not observed in the absence of IPTG or in the control strain (Fig. 5). These results suggest that the AG1/pCA24NgshA strain generates inclusion bodies under biosynthesis conditions, likely because of GshA expression and cadmium stress.
Electron-dense material formation under biosynthesis conditions. (A-D) Representative micrographs of cells from the indicated strains after biosynthesis (CdCl2) or control conditions visualized by TEM. (E & F) Digital zoom of the indicated region in (C) and (D), respectively. (G) Percentage of cells containing electron-dense material of the indicated strains after biosynthesis (CdCl2) or control conditions. The solid-colored line represents the mean value, analyzed from three TEM micrographs. Comparison between groups was analyzed with a Kruskal-Wallis test (p = 0.028)
Inclusion bodies formation in AG1/pCA24N gshA strain. SDS-PAGE analysis of soluble (S) and insoluble (I) proteins from the indicated strains of E. coli with or without (-) IPTG induction. Red rectangle shows the bands corresponding to the induced GshA protein. Std = Protein weight standard. Protein weight is indicated in kDa
Biosynthesized nanoparticles in E. coli are expelled from the cell in minicell-like structures
Ultrastructural analysis of individual cells from the AG1/pCA24NgshA strain under biosynthesis conditions revealed distinct patterns of nanoparticle accumulation, offering insights into potential sequential mechanisms for relocating nanoparticles to the cell poles. Some cells displayed electron-dense material accumulation at both poles (Fig. 6A and B), while others, during cell division, exhibited segregation of this material to one of the daughter cells (Fig. 6 C, 6D). This behavior may explain why certain cells showed QD accumulation at only one pole (Fig. 6E, 6 F), aligning with a cell-aging model in which damaged elements, such as inclusion bodies, are relocated to the poles of E. coli cells for elimination in a daughter cell [31, 45]. Additionally, the observed patterns of electron-dense material accumulation were consistent with fluorescence microscopy images (Fig. 2), further supporting the link between nanoparticles and inclusion bodies.
Nanoparticle localization in E. coli cells. Representative TEM micrographs of strain AG1/pCA24NgshA after biosynthesis conditions, showing the ultrastructure of cells and localization of intracellular nanoparticles. (A & B) Cells showing polar concentration of electron-dense material. (C & D) Cells in division, segregating electron-dense material to a daughter cell. (E & F) Cells with single-pole concentration of electron-dense material
Interestingly, the ultrastructure of the AG1/pCA24NgshA strain under biosynthesis conditions revealed a distinctive minicell-forming phenotype (Fig. 7A, 7 C). These cells exhibited polar division sites, resulting in structures approximately 0.5 μm in size (Fig. 7B and D). Notably, TEM micrographs showed that these structures lacked DNA, with DNA concentrated in the rod-shaped cell. Closer examination identified these structures as containing electron-dense material, previously recognized as inclusion bodies. Within these inclusion bodies, nanometric material, likely corresponding to the synthesized nanoparticles, was observed (Fig. 7B and D). Measurements from TEM micrographs were presented as size histograms, indicating a predominant nanoparticle size range of 5 to 10 nm, consistent with previously reported sizes for CdS nanoparticles [14]. These findings suggest that in the E. coli AG1/pCA24NgshA strain, CdS QD biosynthesis is associated with minicell production.
Discussion
In this study, we conducted an extensive morphological investigation of an E. coli system for the biosynthesis of CdS nanoparticles. Notably, this is the first report highlighting the connection between minicell formation and nanoparticle biosynthesis in E. coli without genetic modifications that promote this phenotype.
The formation of minicells, typically attributed to Min system deletion, was initially considered devoid of a physiological role. However, research by Rang et al. (2018) revealed that an E. coli ΔminC strain exhibited increased tolerance to streptomycin, with minicells playing a role in the disposal of inclusion bodies and misfolded proteins generated by antibiotic exposure [31]. Similarly, a previous study from our research group demonstrated that, in Min-deletion mutants of E. coli conducting intracellular biosynthesis of CdS QDs, nanoparticles were sequestered and expelled from the cells inside the produced minicells, contributing to the maintenance of cellular integrity [33]. These findings indicate a detoxifying mechanism for damaged elements in E. coli cells associated with minicell production, a process that appears to be extended to nanoparticle biosynthesis, which also implies that minicells serve as vehicle for metal disposal from the cell as a detoxifiyng mechanism. While no reports link heavy metal exposure to minicell formation in E. coli, previous studies on the effects of cadmium ions on E. coli cells could provide insights. For instance, cadmium ions exert their primary effects on E. coli cells through inducing misfolded protein stress. This stress is mediated by cadmium’s affinity to sulfide present in cysteine and iron-sulfur centers, forming complexes by binding to thiol groups and replacing other transition-metal cations in such sulfur-rich compounds [46,47,48]. Consequently, this is associated with a negative impact on cell division. Specifically, exposure of E. coli cells to cadmium ions and CdS nanoparticles leads to cell filamentation and hinders the proper formation of the division septum. This inhibition is attributed to a decrease in the expression of key division proteins, FtsZ and FtsQ [49, 50]. These findings suggest that cadmium exposure might induce changes in the expression of cell division proteins, potentially resulting in a minicell-forming phenotype.
The exploration of cell morphology during the biosynthesis of metal nanoparticles has been limited, likely due to the dynamic nature of the process and the predominant focus on biotechnological applications for biological synthesis analysis. In the study by Marusak et al. (2016) [51], it was noted that the addition of CdCl2 and cysteine as a sulfur source at various growth stages of E. coli can influence crystal size and the localization of cadmium precipitation, enabling extracellular biosynthesis. Similarly, multiple studies have documented the extracellular biosynthesis of cadmium nanoparticles through volatile compounds [13, 20, 52], streamlining their preparation, purification, and enhancing the final yield. The relevance of extracellular biosynthesis has supported the study of the biosynthesis process as means of avoiding the toxic effect of cadmium on bacterial cells. For instance, it was demonstrated that in sea-derived bacterial strains, promoting the extracellular biosynthesis of CdS nanoparticles through cysteine supplementation alleviates the toxicity of the metal, and the cell does not need to respond by expressing metal efflux proteins or Reactive oxygen species (ROS) scavenging enzymes [2, 53, 54]. Simillarly, for Shewanella oneidensis, addition of exogenous extracellular polymeric substances (EPS) promotes CdS biosynthesis and enhances cell viability [55]. This behaviour is not limited to cadmium, as metal tolerance and detoxification has also been related to biosynthesis of nanoparticles made of iron [56], copper [57] and terbium [58], just to name a few. However, the same has not been thoroughly investigated in intracellular biosynthesis, so understanding the cellular structures involved in the disposal of nanoparticles could enhance our view of biosynthesis as a resistance mechanism.
In our previous work, we highlighted that in this system, fluorescence emission becomes concentrated in well-defined polar structures, either within the cell or directly associated with the cell poles. This observation was corroborated in this study by analyzing the fluorescence profiles of cells (Fig. 1D). Interestingly, this phenomenon of nanoparticle localization and membrane interaction is not unique to our system but has also been observed in other biosynthesis setups. For example, in a strain expressing a CdS binding peptide, TEM analysis revealed that nanostructures are associated with the cell membrane and poles [59]. Similar behavior was noted by Marusak et al. (2016) [51], where CdS nanoparticles synthesized by an E. coli strain expressing a heterologous cysteine desulfhydrase gene were primarily located near the cell membranes. Furthermore, in an E. coli system designed for the biosynthesis of cadmium and selenium QDs, nanoparticles were predominantly situated at the cell poles [60]. A comparable polar localization of nanoparticles was observed in an E. coli system expressing cystathionine γ-lyase from Stenotrophomonas maltophilia for CdS QD biosynthesis [61]. Notably, this observed phenotype is also present in other Gram-negative bacteria, such as species from the Pseudomonas and Psychrobacter genera. In these bacteria, during the synthesis of CdS QDs, nanomaterials accumulate near the cell poles, accompanied by morphological changes, such as loss of membrane integrity [19] or widening of the periplasmic space [62]. This evidence supports the hypothesis that the process of cell division may be intertwined with QD biosynthesis. These observations led us to hypothesize that QD biosynthesis in bacteria is linked to cell fragmentation or even the generation of minicells. This fragments would in turn, eliminate harmful cadmium ions from the intracelullar space.
Fluorescence microscopy analysis revealed various morphological changes and patterns of fluorescence emission in cells following QD biosynthesis (Fig. 2). Specifically, three primary patterns were observed: fluorescence concentrated at one cell pole (Fig. 2A and B), at both poles (Fig. 2 C, 2D), or randomly distributed within the cells (Fig. 2F and G). These patterns were present in 27%, 53%, and 20% of the population, respectively. Additionally, another behavior was detected, involving the formation of fluorescent spherical cells adjacent to the cell poles (Fig. 2H and I). Although challenging to detect, we cannot rule out the possibility that the resolution of the microscope may not allow for the clear observation of these structures separating from rod cells. If our initial hypothesis is correct, these spherical structures may represent minicells formed as a mechanism for metal disposal, while the polar localization of nanoparticles could signify an intermediate stage in which metals are organized for expulsion. The random distribution of fluorescence emission in some cells may indicate regions of nanoparticle biosynthesis that precede localization at the poles.
Given the different patterns and cell lengths observed through fluorescence microscopy, we hypothesized that CdS QD biosynthesis might be linked to changes in cell length. To explore this, we measured cell length from optical microscopy images under the various conditions tested (Fig. 1A). Both cadmium exposure and overexpression of the GshA protein resulted in significant changes in cell length (Fig. 3A). This effect was more pronounced when cells were clustered into three subgroups, revealing that each treatment was associated with an overall reduction in cell size (Figure S3). Interestingly, when analyzing the cell length distribution following biosynthesis conditions in the AG1/pCA24NgshA strain, we identified a secondary population of cells approximately 1 μm in length (Fig. 3A), consistent with the heterogeneous size distribution observed in minicells [42, 43]. This suggests that this secondary population may represent the formation of minicells under these conditions. Notably, this phenomenon was not observed in the control strain AG1/pCA24N exposed to cadmium, implying that minicell production is dependent on CdS QD biosynthesis and supports their role as detoxifying mechanism. This pattern was further confirmed by analyzing the smaller size clusters, which clearly indicated the emergence of this secondary population under the specified conditions (Fig. 3B).
As previously noted, cadmium exposure in E. coli has been associated with an increase in cell size [49], rather than a decrease. This discrepancy could be due to the markedly different conditions used in our study. For instance, cells were not continuously grown in the presence of cadmium but were instead grown to the stationary phase before being exposed to the metal for an extended period. Additionally, the cadmium concentration used in our study was relatively low compared to other reports. Our findings could represent a form of “equilibrium” between E. coli biomass and cadmium ions and/or nanoparticles.
Furthermore, overexpression of the GshA protein was also associated with a decrease in cell size. A study by Basan et al. (2015) demonstrated that overexpression of a “useless” protein (LacZ) led to an increase in cell length and volume during the exponential growth phase. However, the same study showed that nutrient limitation had the opposite effect [63]. If our “equilibrium” model is correct, we could speculate that the reduction in cell size associated with GshA overexpression may be due to the nutrient-depleted state of cells following protein overexpression. Additionally, it is important to note that the redox activity of the GshA protein is involved in numerous cellular processes in E. coli and other organisms [64, 65], so its overexpression could influence cell size through these processes as well.
Currently, there are no reports documenting minicell formation as a consequence of heavy metal exposure or nanoparticle biosynthesis. However, some studies have investigated gene regulation related to cell division and the expression of proteins in the Min system. For example, in Actinobacillus actinomycetemcomitans, the minC gene is downregulated in the presence of iron via the Fur protein [66]. Similarly, in Neisseria gonorrhoeae, the expression of the minD gene is regulated by the OxyR protein, and cells deficient in OxyR exhibit an increased rate of aberrant, non-midline formation of the division septum compared to wild-type cells [67], a pattern that aligns with our findings (Fig. 3). These precedents suggest that metal exposure in bacteria could induce morphological changes, potentially explaining the formation of minicell-like structures in our system. Additionally, in our system, it is important to consider the possibility that the nutrient-depleted state could be influencing the cellular response to cadmium exposure, thereby promoting minicell formation. However, further research is needed to explore this possibility.
TEM analysis revealed well-defined electron-dense material in cells from the strain AG1/pCA24NgshA after biosynthesis conditions (Fig. 4E, 4 F). Due to the overexpression of the GshA protein in this strain, these structures are likely inclusion bodies. This is substantiated by the higher proportion of cells with this type of electron-dense material in strain AG1/pCA24NgshA compared to the control strain, and the increased proportion after exposure to the metal precursor CdCl2 (Fig. 4G). This suggests that metal exposure is augmenting the number of inclusion bodies, a phenomenon already documented in the literature [46,47,48]. Furthermore, this was corroborated through SDS-PAGE analysis, revealing the presence of part of the GshA protein in the insoluble fraction of proteins in strain AG1/pCA24NgshA after IPTG induction (Fig. 5).
Minicell formation was confirmed through TEM (Fig. 7). Additionally, minicells in formation were found to be loaded with electron-dense inclusion bodies and nanometric material (Fig. 7B and D), providing support for the hypothesis of their role in the disposal of cadmium metal as nanoparticles from the cell. A previous report by Rang et al. (2018) [31] elucidated the role of minicell formation in eliminating misfolded proteins and inclusion bodies from cells. In this model, inclusion bodies relocate to cell poles for encapsulation in minicells, subsequently leading to their expulsion. A similar process could be occurring in our system, where after the relocation of nanoparticles bound to inclusion bodies to poles, they would be encapsulated in minicells for detoxification from the cell.
In strain AG1/pCA24NgshA, electron-dense material localization exhibited similar patterns to those visualized by fluorescence microscopy (Fig. 2), primarily characterized by the polar localization of electron-dense material (Fig. 6A and B). This suggests that biosynthesized nanoparticles are likely bound to the inclusion bodies generated in the process. Previous characterization of these nanoparticles revealed that they were GSH-capped [14]. Additionally, to control the intracellular amount of GSH, the GshA enzyme is regulated by GSH, which acts as a competitive inhibitor by binding to GshA’s cysteine-binding site [68, 69]. Hence, it is plausible that QDs binding to excess GSH, generated by the overexpression of GshA, are also binding to the GshA protein forming inclusion bodies. Several reports on bacterial QD synthesis suggest that these nanoparticles exhibit a capping made of organic matter, proteins, and other biomolecules, playing a role in nanoparticle formation, stabilization, and overall properties [13, 14, 70, 71]. Therefore, the interaction between inclusion bodies and nanoparticles during the biosynthesis process is plausible. Moreover, studies have reported the preferential polar localization of various proteins, including chaperones such as IbpA [31] and GroEL [72]. Chaperones have been proposed to play a role in the synthesis of nanoparticles [70], and their ability to relocate within the cell could contribute to the polar accumulation of inclusion bodies and nanoparticles. Additionally, new protein factors in E. coli have been described for the polar localization of proteins, such as TmaR, a protein involved in the polar localization of components of the phosphotransferase system [73]. Furthermore, other proteins have been reported to “mark” spots for division sites that eventually turn into poles, as demonstrated by chemotaxis receptors in E. coli, which form clusters in division sites two generations before actual division [74].
Another observation made through TEM was the segregation of inclusion bodies to daughter cells following cell division (Fig. 6C and D), which could explain the accumulation of electron-dense material in a single cell pole (Fig. 6E, 6 F). This phenomenon aligns with the cell aging model proposed by Shi et al. (2020), wherein older poles accumulate damaged elements. After cell division, one daughter cell, referred to as the “old daughter”, inherits these elements and exhibits slower growth, preserving the fitness of the “new” daughter [45]. In our system, a similar scenario may occur, where misfolded proteins and metal nanoparticles synthesized during biosynthesis are relocated to the cell poles. Upon division, these components would be retained in the old daughter cell, facilitating the production of metal-free cells. This mechanism suggests a potential strategy by which cells rid themselves of damaged or harmful components, thereby ensuring the vitality of newly formed cells. Interestingly, we observed minicell formation only in cells with electron-dense material accumulated at a single pole (Fig. 7), but not in those with accumulation at both poles. This might indicate that for minicell formation to occur, the cell must be in a favorable state. A division step resulting in single-pole accumulation could be a prerequisite for promoting minicell formation, though further research is required to confirm this hypothesis.
Our results prompt the question of how minicells are generated in some cells in response to QDs biosynthesis. The low proportion of cells generating minicells under CdS nanoparticles biosynthesis conditions (Fig. 3) suggests that this is not a physiologically promoted mechanism, especially considering the apparent need for GshA overexpression for its occurrence. In the study by Hoffman and Frank (1963) [75], exposure of various E. coli cultures to increased temperature (43.5 °C) resulted in only one minicell out of “several thousand” cells. This underscores the challenge of studying this phenomenon in cells lacking mutations in the Min system. Regarding the molecular mechanism for minicell formation, it could be mediated by a general down-regulation of genes involved in cell division as a consequence of cadmium exposure [76]. As mentioned earlier, exposure of E. coli cells to cadmium ions and CdS nanoparticles induces cell filamentation and inhibits the correct formation of the division septum, involving a decrease in the expression of FtsZ and FtsQ division proteins [49, 50]. These proteins play a role in the formation of the “Z-ring” in the center of the cell [77]. Additionally, the Min system finely regulates cell division location, and E. coli cells deficient in it exhibit high filamentation and divide in random spots, leading to the formation of minicells [30]. Furthermore, down-regulation of other components of the E. coli divisome could also contribute to this phenomenon. Unpublished results from our group indicate a decrease in the expression of the ZapB protein under CdS QDs biosynthesis, and the deletion of this protein, along with lower expression of FtsZ, induces the minicell formation phenotype [78], providing another potential explanation for this occurrence.
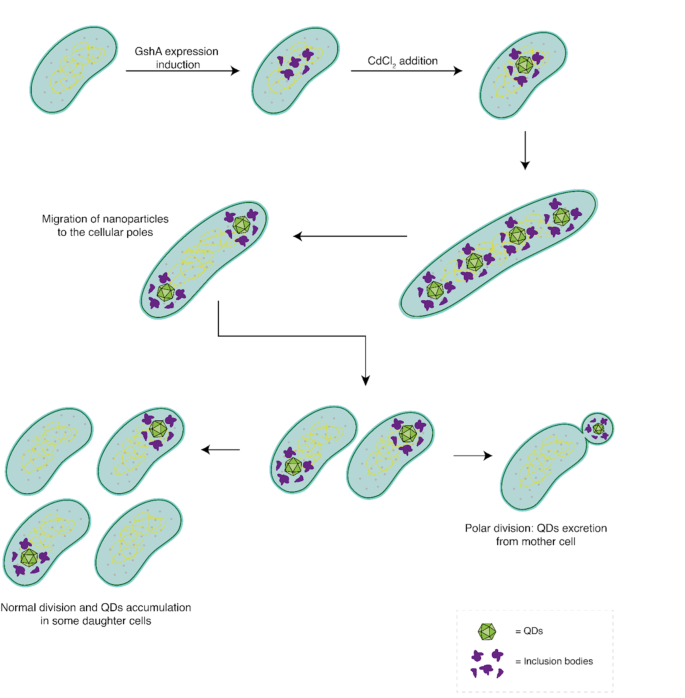
Our results, combined with previous characterizations of the system [14, 33], enable us to propose a model illustrating the various morphological changes that occur in strain AG1/pCA24NgshA following the biosynthesis of CdS nanoparticles (Fig. 8). The process begins with the induction of GshA protein expression by IPTG, leading to an increase in intracellular GSH concentration and the formation of inclusion bodies. Subsequently, the addition of the metal precursor to the culture media initiates nanoparticle biosynthesis throughout the cytosol. These nanoparticles bind to the inclusion bodies, either via the GSH cap or through interactions with chaperone proteins. The synthesized nanoparticles are then relocated to both cell poles as an intermediate cellular form. Following this, the cell resumes normal division, allowing for the segregation of QDs into daughter cells. At this point, two possibilities arise: (1) Through successive cell divisions, QD-free cells are generated, which can proliferate normally. (2) If a cell experiences down-regulation of factors involved in septum localization, polar division may occur, resulting in the formation of a minicell that encapsulates and disposes of the metal from the cell.
Morphologic model of cadmium nanoparticles biosynthesis in E. coli AG1/pCA24N gshA. First, the GshA protein is overexpressed in the AG1/pCA24NgshA strain, promoting the formation of inclusion bodies. Subsequently, metal precursors are incorporated into the cell, where nanoparticles are synthesized by the action of intracellular GSH. These nanoparticles, associated with inclusion bodies, are then relocated to the cell poles. Following this, cell division occurs, resulting in cells with a single cell pole containing an accumulation of QDs. Normal division can continue, leading to the sequential accumulation of QDs in some cells while allowing the formation of cadmium-free cells. Alternatively, polar division may take place, generating a minicell that encapsulates the nanoparticles and separates them from the main cell
Conclusion
This study provides a comprehensive analysis of E. coli morphology in a defined system for the biosynthesis of CdS nanoparticles. Our findings reveal that nanoparticles predominantly accumulate in cell poles, triggering significant changes in cell division, filamentation, and the formation of minicells for nanoparticle disposal. To our knowledge, this is the first report linking metal exposure, nanoparticle biosynthesis, and minicell production in E. coli without genetic modifications that directly promote minicell formation. This discovery opens a new dimension in our comprehension of bacterial responses to heavy metals and offers insights into how nanoparticle expulsion from the cell can be optimized for biotechnological applications.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- LB:
-
Lysogeny broth
- CdS:
-
Cadmium sulphide
- CdTe:
-
Cadmium telluride
- GshA:
-
L-glutamate-cysteine ligase
- GSH:
-
Glutathione
- H2S:
-
Hydrogen sulfide
- IPTG:
-
Isopropyl-b-D-1-thiogalactopyranoside
- PMSF:
-
phenylmethylsulfonyl fluoride
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TEM:
-
Transmission electron microscopy
- QDs:
-
Quantum dots
- NA:
-
Numerical aperture
- ROS:
-
Reactive oxygen species
- EPS:
-
Extracellular polymeric substances
References
Turner RJ, Huang L-N, Viti C, Mengoni A. Metal-Resistance Bacteria: Why Care? Genes. 2020;11:1470.
Ma N, Cai R, Sun C. Threonine dehydratase enhances bacterial cadmium resistance via driving cysteine desulfuration and biomineralization of cadmium sulfide nanocrystals. J Hazard Mater. 2021;417:126102.
Mansur HS. Quantum dots and nanocomposites. WIREs Nanomed Nanobiotechnol. 2010;2:113–29.
Jahangir MA, Gilani SJ, Muheem A, Jafar M, Aslam M, Ansari MT, et al. Quantum dots: Next Generation of Smart Nano-Systems. PNT. 2019;7:234–45.
Sumanth Kumar D, Jai Kumar B, Mahesh HM. Quantum Nanostructures (QDs): An Overview. Synthesis of Inorganic Nanomaterials [Internet]. Elsevier; 2018 [cited 2024 Sep 24];59–88. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780081019757000038
Pleskova S, Mikheeva E, Gornostaeva E. Using of Quantum Dots in Biology and Medicine. In: Saquib Q, Faisal M, Al-Khedhairy AA, Alatar AA, editors. Cellular and Molecular Toxicology of Nanoparticles [Internet]. Cham: Springer International Publishing; 2018 [cited 2024 Sep 24]. pp. 323–34. Available from: http://link.springer.com/https://doi.org/10.1007/978-3-319-72041-8_19
Crisp RW, Pach GF, Kurley JM, France RM, Reese MO, Nanayakkara SU, et al. Tandem Solar cells from solution-processed CdTe and PbS Quantum dots using a ZnTe–ZnO tunnel Junction. Nano Lett. 2017;17:1020–7.
Abbasi S, Molaei M, Karimipour M. CdSe and CdSe/CdS core–shell QDs: New approach for synthesis, investigating optical properties and application in pollutant degradation. Luminescence. 2017;32:1137–44.
Yin J, Cogan NMB, Burke R, Hou Z, Sowers KL, Krauss TD. Size dependence of photocatalytic hydrogen generation for CdTe quantum dots. J Chem Phys. 2019;151:174707.
Wegner KD, Resch-Genger U. The 2023 Nobel Prize in Chemistry: Quantum dots. Anal Bioanal Chem. 2024;416:3283–93.
Hulkoti NI, Taranath TC. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf B. 2014;121:474–83.
Saravanan A, Kumar PS, Karishma S, Vo D-VN, Jeevanantham S, Yaashikaa PR, et al. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere. 2021;264:128580.
Bao H, Lu Z, Cui X, Qiao Y, Guo J, Anderson JM, et al. Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater. 2010;6:3534–41.
Monrás JP, Díaz V, Bravo D, Montes RA, Chasteen TG, Osorio-Román IO et al. Enhanced Glutathione Content Allows the In Vivo Synthesis of Fluorescent CdTe Nanoparticles by Escherichia coli. Bansal V, editor. PLoS ONE. 2012;7:e48657.
Van Veen HW, Abee T, Kortstee GJJ, Konings WN, Zehnder AJB. Translocation of metal phosphate via the Phosphate Inorganic Transport System of Escherichia coli. Biochemistry. 1994;33:1766–70.
Martín JF, Liras P. Molecular mechanisms of phosphate sensing, Transport and Signalling in Streptomyces and related Actinobacteria. IJMS. 2021;22:1129.
Venegas FA, Saona LA, Monrás JP, Órdenes-Aenishanslins N, Giordana MF, Ulloa G, et al. Biological phosphorylated molecules participate in the biomimetic and biological synthesis of cadmium sulphide quantum dots by promoting H 2 S release from cellular thiols. RSC Adv. 2017;7:40270–8.
Bai HJ, Zhang ZM, Guo Y, Yang GE. Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas Palustris. Colloids Surf B. 2009;70:142–6.
Gallardo C, Monrás JP, Plaza DO, Collao B, Saona LA, Durán-Toro V, et al. Low-temperature biosynthesis of fluorescent semiconductor nanoparticles (CdS) by oxidative stress resistant Antarctic bacteria. J Biotechnol. 2014;187:108–15.
Órdenes-Aenishanslins N, Anziani-Ostuni G, Monrás JP, Tello A, Bravo D, Toro-Ascuy D, et al. Bacterial synthesis of Ternary CdSAg Quantum dots through Cation Exchange: tuning the Composition and properties of Biological nanoparticles for Bioimaging and Photovoltaic Applications. Microorganisms. 2020;8:631.
Wang Y, Liu Y, Bai L, Wang J, Zhao N, Cui D, et al. Low-toxicity self-photosensitized Biohybrid systems for enhanced Light-Driven H2 production. IJMS. 2024;25:3085.
Qin Z, Yue Q, Liang Y, Zhang J, Zhou L, Hidalgo OB, et al. Extracellular biosynthesis of biocompatible cadmium sulfide quantum dots using Trametes Versicolor. J Biotechnol. 2018;284:52–6.
Xu J, Hu R, Wang Q, Wang P, Bao H. Extracellular biosynthesis of biocompatible CdSe quantum dots. IET Nanobiotechnol. 2019;13:962–6.
Gangan MS, Naughton KL, Boedicker JQ. Utilizing a divalent metal ion transporter to control biogenic nanoparticle synthesis. J Ind Microbiol Biotechnol. 2023;50:kuad020.
Li Y, Wei G, Chen J. Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol. 2004;66:233–42.
Adler HI, Fisher WD, Cohen A, Hardigree AA. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci USA. 1967;57:321–6.
Pichoff S, Vollrath B, Touriol C, Bouché J. Deletion analysis of gene minE which encodes the topological specificity factor of cell division in Escherichia coli. Mol Microbiol. 1995;18:321–9.
Yu X-C, Margolin W. Deletion of the min Operon results in increased thermosensitivity of an ftsZ84 mutant and abnormal FtsZ Ring Assembly, Placement, and Disassembly. J Bacteriol. 2000;182:6203–13.
Ramm B, Heermann T, Schwille P. The E. Coli MinCDE system in the regulation of protein patterns and gradients. Cell Mol Life Sci. 2019;76:4245–73.
Wehrens M, Ershov D, Rozendaal R, Walker N, Schultz D, Kishony R, et al. Size laws and Division Ring dynamics in Filamentous Escherichia coli cells. Curr Biol. 2018;28:972–e9795.
Rang CU, Proenca A, Buetz C, Shi C, Chao L. Minicells as a Damage Disposal Mechanism in Escherichia coli. Bowman GR, editor. mSphere. 2018;3:e00428-18.
Kim S-J, Oh M-K. Minicell-forming Escherichia coli mutant with increased chemical production capacity and tolerance to toxic compounds. Bioresour Technol. 2023;371:128586.
Valenzuela-Ibaceta F, Torres-Olea N, Ramos-Zúñiga J, Dietz-Vargas C, Navarro CA, Pérez-Donoso JM. Minicells as an Escherichia coli mechanism for the accumulation and disposal of fluorescent cadmium sulphide nanoparticles. J Nanobiotechnol. 2024;22:78.
Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, et al. Complete set of ORF clones of Escherichia coli ASKA library (A Complete Set of E. Coli K-12 ORF archive): Unique resources for Biological Research. DNA Res. 2006;12:291–9.
Laemmli UK. Cleavage of structural proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680–5.
Ramírez MP, Rivera M, Quiroga-Roger D, Bustamante A, Vega M, Baez M, et al. Single molecule force spectroscopy reveals the effect of BiP chaperone on protein folding. Protein Sci. 2017;26:1404–12.
Gallagher (Electrophoresis S. Sasse (Staining) J. Protein Analysis by SDS-PAGE and Detection by Coomassie Blue or Silver Staining. CP Pharmacology [Internet]. 1998 [cited 2024 Sep 24];2. Available from: https://currentprotocols.onlinelibrary.wiley.com/doi/https://doi.org/10.1002/0471141755.pha03bs02
Jain AK, Dubes RC. Algorithms for clustering data. Englewood Cliffs, N.J: Prentice Hall; 1988.
Watanabe K, Yamano Y, Murata K, Kimura A. The nudeotide sequence of the gene for γ-glutamylcysteine synthetase of Escherichia coli. Nucl Acids Res. 1986;14:4393–400.
Pérez-Donoso JM, Monrás JP, Bravo D, Aguirre A, Quest AF, Osorio-Román IO et al. Biomimetic, Mild Chemical Synthesis of CdTe-GSH Quantum Dots with Improved Biocompatibility. Bansal V, editor. PLoS ONE. 2012;7:e30741.
Zhang Y, Chen Q, Chen S, Wang S, Zhang M, Yu K. Evolution of aqueous-phase CdTe magic-size clusters from their precursor compounds. J Phys Chem Lett. 2023;14:5188–93.
Kobayashi H. Regeneration of Escherichia coli from Minicells through Lateral Gene Transfer. DiRita VJ, editor. J Bacteriol [Internet]. 2018 [cited 2024 Sep 24];200. Available from: https://doi.org/10.1128/JB.00630-17
Burt A, Cassidy CK, Ames P, Bacia-Verloop M, Baulard M, Huard K, et al. Complete structure of the chemosensory array core signalling unit in an E. coli minicell strain. Nat Commun. 2020;11:743.
Farley MM, Hu B, Margolin W, Liu J, Minicells. Back in Fashion. De Boer P, editor. J Bacteriol. 2016;198:1186–95.
Shi C, Chao L, Proenca AM, Qiu A, Chao J, Rang CU. Allocation of gene products to daughter cells is determined by the age of the mother in single Escherichia coli cells. Proc R Soc B. 2020;287:20200569.
Helbig K, Grosse C, Nies DH. Cadmium toxicity in glutathione mutants of Escherichia coli. J Bacteriol. 2008;190:5439–54.
Sharma SK, Goloubinoff P, Christen P. Heavy metal ions are potent inhibitors of protein folding. Biochem Biophys Res Commun. 2008;372:341–5.
Cornejo FA, Muñoz-Villagrán C, Luraschi RA, Sandoval-Díaz MP, Cancino CA, Pugin B, et al. Soft-metal(loid)s induce protein aggregation in Escherichia coli. Front Microbiol. 2023;14:1281058.
Hossain ST, Mallick I, Mukherjee SK. Cadmium toxicity in Escherichia coli: cell morphology, Z-ring formation and intracellular oxidative balance. Ecotoxicol Environ Saf. 2012;86:54–9.
Hossain ST, Mukherjee SK. Toxicity of cadmium sulfide (CdS) nanoparticles against Escherichia coli and HeLa cells. J Hazard Mater. 2013;260:1073–82.
Marusak KE, Feng Y, Eben CF, Payne ST, Cao Y, You L, et al. Cadmium sulphide quantum dots with tunable electronic properties by bacterial precipitation. RSC Adv. 2016;6:76158–66.
Bruna N, Collao B, Tello A, Caravantes P, Díaz-Silva N, Monrás JP, et al. Synthesis of salt-stable fluorescent nanoparticles (quantum dots) by polyextremophile halophilic bacteria. Sci Rep. 2019;9:1953.
Ma N, Sha Z, Sun C. Formation of cadmium sulfide nanoparticles mediates cadmium resistance and light utilization of the deep-sea bacterium Idiomarina sp. OT37 ‐5b. Environ Microbiol. 2021;23:934–48.
Ma N, Sun C. Cadmium sulfide nanoparticle biomineralization and biofilm formation mediate cadmium resistance of the deep-sea bacterium Pseudoalteromonas sp. MT33b. Environ Microbiol Rep. 2021;13:325–36.
Zhang S, Li C, Ke C, Liu S, Yao Q, Huang W, et al. Extracellular polymeric substances sustain photoreduction of cr(VI) by Shewanella oneidensis-CdS biohybrid system. Water Res. 2023;243:120339.
Chen J, Gan L, Han Y, Owens G, Chen Z. Ferrous sulfide nanoparticles can be biosynthesized by sulfate-reducing bacteria: synthesis, characterization and removal of heavy metals from acid mine drainage. J Hazard Mater. 2024;466:133622.
Kimber RL, Bagshaw H, Smith K, Buchanan DM, Coker VS, Cavet JS et al. Biomineralization of Cu 2 S Nanoparticles by Geobacter sulfurreducens. Nojiri H, editor. Appl Environ Microbiol. 2020;86:e00967-20.
León JJ, Oetiker N, Torres N, Bruna N, Oskolkov E, Lei P, et al. Microbial green synthesis of luminescent terbium sulfide nanoparticles using E. Coli: a rare earth element detoxification mechanism. Microb Cell Fact. 2024;23:248.
Mi C, Wang Y, Zhang J, Huang H, Xu L, Wang S, et al. Biosynthesis and characterization of CdS quantum dots in genetically engineered Escherichia coli. J Biotechnol. 2011;153:125–32.
Zhu T-T, Tian L-J, Yu S-S, Yu H-Q. Roles of cation efflux pump in biomineralization of cadmium into quantum dots in Escherichia coli. J Hazard Mater. 2021;412:125248.
Niu L, Yu L, Jin C, Jin K, Liu Z, Zhu T, et al. Living materials based dynamic information encryption via Light-Inducible Bacterial Biosynthesis of Quantum Dots. Angew Chem Int Ed. 2024;63:e202315251.
Plaza DO, Gallardo C, Straub YD, Bravo D, Pérez-Donoso JM. Biological synthesis of fluorescent nanoparticles by cadmium and tellurite resistant Antarctic bacteria: exploring novel natural nanofactories. Microb Cell Fact. 2016;15:76.
Basan M, Zhu M, Dai X, Warren M, Sévin D, Wang Y, et al. Inflating bacterial cells by increased protein synthesis. Mol Syst Biol. 2015;11:836.
Apontoweil P, Berends W. Mapping of gshA, a gene for the biosynthesis of glutathione in Eschericha Coli K12. Molec Gen Genet. 1975;141:91–5.
Van Laar TA, Esani S, Birges TJ, Hazen B, Thomas JM, Rawat M. Pseudomonas aeruginosa gshA Mutant Is Defective in Biofilm Formation, Swarming, and Pyocyanin Production. D’Orazio SEF, editor. mSphere. 2018;3:e00155-18.
Haraszthy VI, Jordan SF, Zambon JJ. Identification of Fur-regulated genes in Actinobacillus actinomycetemcomitans. Microbiology. 2006;152:787–96.
Parti RP, Horbay MA, Liao M, Dillon J-AR. Regulation of minD by oxyR in Neisseria gonorrhoeae. Res Microbiol. 2013;164:406–15.
Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975;250:1422–6.
Hibi T, Nii H, Nakatsu T, Kimura A, Kato H, Hiratake J, et al. Crystal structure of γ-glutamylcysteine synthetase: insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis. Proc Natl Acad Sci USA. 2004;101:15052–7.
Ishii D, Kinbara K, Ishida Y, Ishii N, Okochi M, Yohda M, et al. Chaperonin-mediated stabilization and ATP-triggered release of semiconductor nanoparticles. Nature. 2003;423:628–32.
Bruna N, Galliani E, Oyarzún P, Bravo D, Fuentes F, Pérez-Donoso JM. Biomineralization of lithium nanoparticles by Li-resistant Pseudomonas rodhesiae isolated from the Atacama salt flat. Biol Res. 2022;55:12.
Li G, Young KD. Isolation and identification of new inner membrane-associated proteins that localize to cell poles in Escherichia coli. Mol Microbiol. 2012;84:276–95.
Szoke T, Albocher N, Govindarajan S, Nussbaum-Shochat A, Amster-Choder O. Tyrosine phosphorylation-dependent localization of TmaR that controls activity of a major bacterial sugar regulator by polar sequestration. Proc Natl Acad Sci USA. 2021;118:e2016017118.
Thiem S, Kentner D, Sourjik V. Positioning of chemosensory clusters in E. Coli and its relation to cell division. EMBO J. 2007;26:1615–23.
Hoffman H, Frank ME, TIME-LAPSE PHOTOMICROGRAPHY OF THE FORMATION OF. A FREE SPHERICAL GRANULE IN AN ESCHERICHIA COLI CELL END. J Bacteriol. 1963;86:1075–8.
Wang A, Crowley DE. Global gene expression responses to Cadmium Toxicity in Escherichia coli. J Bacteriol. 2005;187:3259–66.
Dai K, Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol. 1992;174:6145–51.
Galli E, Gerdes K. FtsZ-ZapA-ZapB interactome of Escherichia coli. J Bacteriol. 2012;194:292–302.
Acknowledgements
This work was supported by Erika Elcira Donoso Lopez and Fondecyt 1200870 (JMP-D). FV-I would like to thank Nicolás Torres-Olea for helping with figure design, and Valentina Carrasco, Eduardo Lamoza-Galleguillos and Microbial Data Science Lab from Universidad Andrés Bello for their help and suggestions with R software and analysis of the data. In the loving memory of Claudio Vásquez Guzmán, an excellent friend, mentor, and scientist, but an even better human being. Thanks for all the adventures and for showing us the beauty of science and friendship.
Funding
This study was financially supported by Fondecyt 1200870 (JMP-D) and AFOSR FA9550-22-1-0509 (JMP-D).
Author information
Authors and Affiliations
Contributions
FV-I: conceptualization, methodology, validation, investigation, writing—original draft, writing—review and editing, visualization; SAA: validation, writing—review and editing. JMP-D: conceptualization, validation, investigation, resources, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Valenzuela-Ibaceta, F., Álvarez, S.A. & Pérez-Donoso, J.M. Production of minicell-like structures by Escherichia coli biosynthesizing cadmium fluorescent nanoparticles: a novel response to heavy metal exposure. J Nanobiotechnol 23, 111 (2025). https://doi.org/10.1186/s12951-025-03188-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-025-03188-2