Abstract
Temporomandibular joint disorder (TMD) has a multifactorial etiology involving psychological and genetic aspects. This condition commonly begins in adolescence, which is a period of emotional, physical, and psychological maturation. The aim of the present study was to investigate associations between temporomandibular joint (TMJ) disorder and happiness as well as polymorphisms in the COMT, HTR2A, and FKBP5 genes in Brazilian adolescents. A cross-sectional study was conducted with 90 adolescents aged 13 to 18 years. TMD was diagnosed using the RDC/TMD. The Subjective Happiness Scale (SHS) was used to assess happiness. Saliva samples were collected for the analysis of genomic DNA and genotyping of single nucleotide polymorphisms in the COMT (rs165656, rs174675), HTR2A (rs6313, rs4941573), and FKBP5 (rs1360780, rs3800373) genes using real-time PCR (Taqman method). Bivariate, unadjusted, and adjusted binary logistic regression analyses were performed (p < 0.05). Happiness was associated with TMD in the adolescents (OR=1.37; 95%CI: 1.02–1.85; p = 0.037). The rs174675 polymorphism in the COMT gene was significantly associated with TMD (OR = 0.18; 95%CI: 0.04–0.74; p = 0.018). No associations were found between TMD and polymorphisms in HTR2A and FKBP5 genes (p > 0.05). TMD was associated with happiness, as adolescents who considered themselves less happy were more likely to have this disorder. The diagnosis of TMD was also associated with the rs174675 polymorphism in the COMT gene, as the prevalence of the disorder was higher among homozygous C Brazilian adolescents than in heterozygous CT individuals.
Temporomandibular Joint Disorders; Happiness; Polymorphism, Genetic; Adolescent
Introduction
Temporomandibular joint disorder (TMD) describes a set of skeletal and neuromuscular conditions in the craniofacial region1,2and is the most common cause of non-dental orofacial pain.2 It may have different diagnoses classified as muscular disorders (myofascial pain or myofascial pain with limited mouth opening), disc displacements (disc displacement which may be with or without reduction), and joint disorders (arthralgia, osteoarthritis, or osteoarthrosis).1,3
The etiology of TMD is complex. The multifactorial biopsychosocial model of TMD is currently the most widely accepted etiology, in which biological, psychological, and social factors act together.3,4 Emotional factors, such as stress, anxiety, and depression, play an important role in the origin and evolution of symptoms.2,4,5 Cohort studies involving adults and adolescents have demonstrated that depression, perceived stress, bad mood, somatization, and dissatisfaction with life increase the risk of TMD and the initiation or perpetuation of pain related to this disorder.6,7
TMD can begin in childhood and adolescence.8,9 We hypothesize that the onset of TMD is influenced by the characteristics of adolescence, which is a period of emotional, pubertal, psychological, and social maturation when individuals are more vulnerable to the emergence of psychopathologies due to increased emotional intensity and immature cognitive control.8,9
The dynamics of TMD are modulated by genetic factors, with gene-environment interactions involved in the emergence of the disorder.4,10,11 Genetic association studies have investigated the role of polymorphisms in the etiology of TMD. The findings indicate several candidate genes that have small effects and determine the course and outcome of the disorder.10,11,12 While such studies have interesting results, the exact genes and polymorphisms associated with risk of or protection from TMD remain unknown.4 Genes involved in the regulation of the serotonergic system have attracted attention due to their role in nociceptive and affective pathways.12Polymorphisms in the catechol-O-methyltransferase (COMT), 5-hydroxytryptamine receptor 2A (HTR2A), and FKBP prolyl isomerase 5 (FKBP5) genes have a potential association with this system.1,4,12,13
The COMT gene encodes the catechol-O-methyltransferase enzyme, which catalyzes the degradation and reuptake of a wide range of catechols, including catecholamines (dopamine, adrenaline, and noradrenaline).4,14,15 Polymorphisms in this gene are associated with emotional disorders,16 anxiety,17 and the pathophysiology of TMD.4,12,17 The HTR2A gene encodes the serotonin receptor 5-hydroxytryptamine, which is an important central nervous system neurotransmitter that regulates various physiological and cognitive functions.18,19 Its variants have been associated with TMD.12,18 bruxism.20 obstructive sleep apnea,21 and psychiatric disorders.22 The FKBP prolyl isomerase 5 (FKBP5) gene plays a role in the sensitivity of the glucocorticoid receptor and therefore regulates the stress response system.23,24 FKBP5 polymorphisms are associated with risk of developing post-traumatic stress disorder,25 depression,23,24 anxiety,23 and greater surgical discomfort during third molar extractions.24
Based on the notion that adolescence influences susceptibility to the emergence of emotional disorders and impacts happiness8,9 and because the first signs and symptoms of TMD often appear in childhood and adolescence, the aim of the present study was to investigate associations between temporomandibular joint disorder (TMJ) happiness, and polymorphisms in the COMT (rs165656, rs174675), HTR2A (rs6313, rs4941573), and FKBP5 (rs1360780, rs3800373) genes in adolescents. Studies involving this period of life can contribute to a better understanding of the onset and evolution of TMD.26,27 To date, however, few have investigated the role of these polymorphisms in the etiology of TMJ among adolescents.4
Methods
Ethics approval
This study was conducted in accordance with the STREGA statement (https://www.equator-network.org/reporting-guidelines/strobe-strega/) and ethical precepts laid down in the Declaration of Helsinki. The study was approved by the Human Research Ethics Committee of the Federal University of Minas Gerais (Protocol #01936918.8.0000.5149). Parents/caregivers or adolescents aged 18 years received written information on the study and signed as statement of informed consent. Adolescents under 18 years of age signed a statement of informed acceptance.
Study population
A cross-sectional study was conducted with a sample of 90 Brazilian adolescents aged 13 to 18 years. Biologically unrelated male and female individuals with or without TMJ were included. Adolescents undergoing orthodontic treatment and those with syndromic or cognitive disorders were excluded.
Data collection
Two examiners (A.L.P.B. and G.A.F.) who had undergone training and calibration performed the clinical examinations and administered the instruments. Training and calibration were conducted by researchers experienced in the use of the clinical indexes employed in this study. The examiners received theoretical and practical training involving clinical examination of 28 patients. Inter-examiner and intra-examiner agreement (kappa coefficient) for the diagnosis of TMJ was 0.907 and 0.804, respectively.
Prior to the main study, a pilot study was conducted with 10 adolescents to test the proposed methods. Based on the pilot study, the methods were maintained and the individuals who participated in this stage were included in the main study.
Data collection took place between May and December 2019. The participants were recruited at the dental clinic of the Federal University of Minas Gerais.
A self-administered questionnaire was developed for this research based on items used in similar studies. There were questions about the adolescents’ socioeconomic and demographic data (name, age, birthday, sex) and oral health-related data (previous dental trauma, sleep and awake bruxism). Then the adolescents were examined in a dental chair under artificial light by an examiner wearing personal protective equipment and using a sterilized clinical kit consisting of a mouth mirror and WHO dental probe. The examiners looked for signs of tooth wear and dental trauma. Positive signs of tooth wear and a parental report of teeth grinding and clenching during the day or night were considered indicative of probable awake or sleep bruxism, respectively. A questionnaire addressing socioeconomic, demographic, and health-related characteristics was administered to the adolescents.
The diagnosis of TMJ was performed using Axis I of the validated Brazilian version of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) to detect muscle disorder, disc displacement, and/or joint disorder29. We focused on joint disorders in the present study. Thus, adolescents with arthralgia, osteoarthritis, and osteoarthrosis (separately or concomitantly) were considered to have TMD. We did not use the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) tool because it had not yet been validated in Brazilian Portuguese at the time of data collection.
The participants also answered the Brazilian version of the Subjective Happiness Scale (SHS),29,30 which is a self-report measure of subjective global happiness. The four-item SHS assesses whether the respondents consider themselves happy or unhappy based the number marked on a seven-point scale indicating the extent to which each of the four statements applies to them. The scale increases from one to seven. A higher score on the first three items indicates a greater degree of happiness, whereas a higher score on the last item indicates a lower degree of unhappiness.29,30
DNA samples and genotyping
DNA was analyzed for genotyping. The adolescents rinsed their mouths with 5 mL of a 3% glucose solution for one minute and then spat the liquid into Falcon tubes to obtain cells from the oral epithelium. 15-mL centrifuge tubes (Corning Inc, Corning, USA) were filled with expectorated saliva samples. The protocol established by Kuchler et al.31 was followed for the extraction of DNA.
The selection of polymorphisms in genes was performed based on previous candidate-gene identification studies10,12,18,24 for the pathophysiology of TMD. Gene characterization and polymorphisms are described in Table 1. Polymorphisms in the COMT (rs165656, rs174675), HTR2A (rs6313, rs4941573), and FKBP5 (rs1360780, rs3800373) genes were determined using polymerase chain reaction (PCR) analysis, employing TaqMan method in a real-time PCR system (Applied Byosistems®, 7500 Real-Time PCR System, Thermo Fisher Scientific, Foster City, USA).
Statistical analysis
The characterization of the sample was performed using descriptive statistics. Unadjusted and adjusted binary logistic regression models were run between the outcome (TMJ) and variables of interest considering the underlying theoretical framework. Variables with a p-value < 0.2 in the bivariate analysis were incorporated into the multivariate model. Variables were also incorporated taking into account the theoretical framework. Wald’s backward method was used to create the final model, generating adjusted odds ratios (OR) and respective 95% confidence intervals (CI) for TMJ according to categories of the independent variables. A p-value < 0.05 was considered indicative of statistical significance. Data analysis was performed with the Microsoft Excel and Statistical Package for the Social Sciences (SPSS, version 22.0, IBM Corp., Armonk, USA).
Results
Descriptive statistics
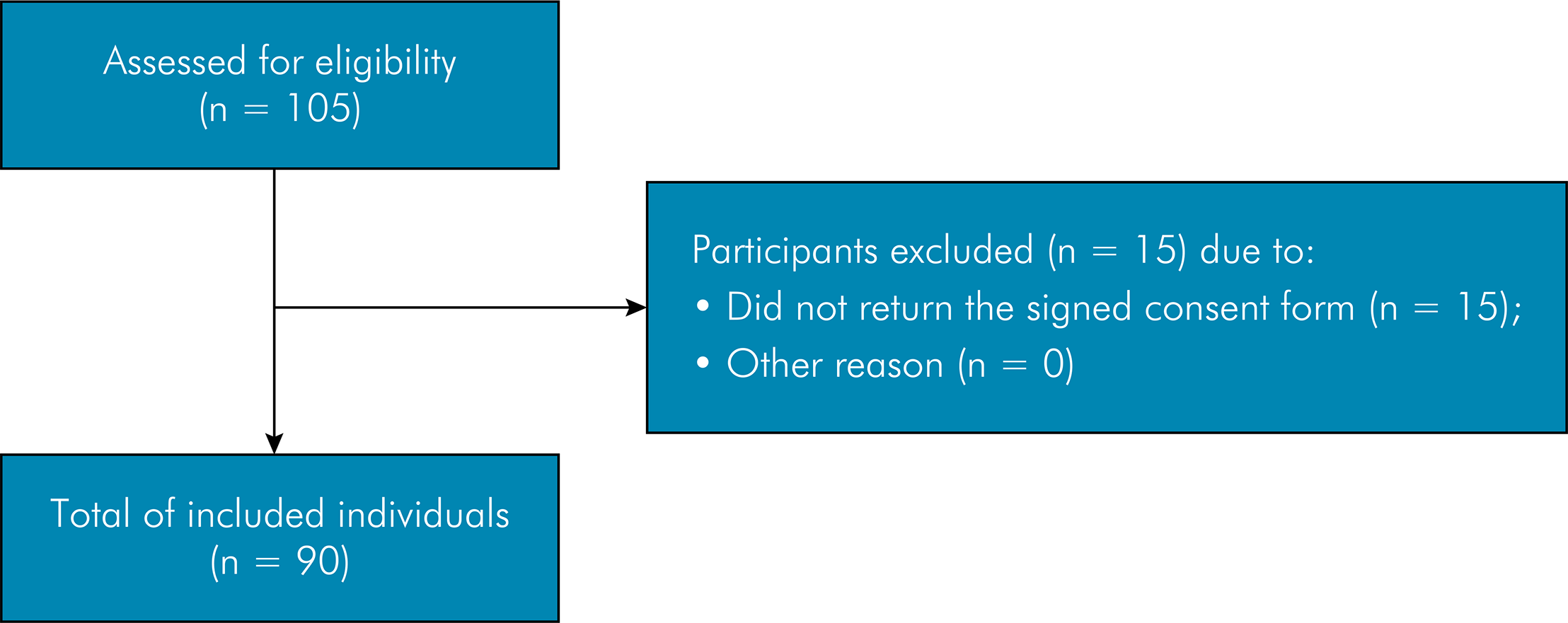
One hundred and five adolescents were recruited for the study, 90 of whom returned the signed informed consent, agreed to participate, and underwent all phases of the study (response rate: 85.7%) (Figure). Forty-six (51.1%) participants were girls and 44 (48.9%) were boys. Mean age was 15.9 years, 73% were non-white and 27% were white, 36% of parents/caregivers had studied 8 years or less, 30.5% of the adolescents’ families received up to 1 Brazilian minimum wage per month (exchange rate was 1 to US$ 196,84 at the time of data collection), and 56.1% received 1 to 3 Brazilian minimum wages per month. The prevalence of TMJ was 41.1% (n = 37). Twenty-three participants with symptoms of TMJ (62.2%) were girls and 14 (37.8%) were boys. Table 2 displays the frequency of each variable.
Analytical statistics
In the binary logistic regression, the fourth item on the Subjective Happiness Scale was associated with TMJ (p = 0.022). For every point higher on the SHS, the risk of TMJ increases by 36%. The rs174675 recessive C COMT gene polymorphism (p = 0.011) and probable sleep bruxism (p = 0.038) were also associated with TMJ in Brazilian adolescents in the binary logistic regression (Table 3).
After adjusting for other variables in the multiple regression analysis (Table 4), TMJ was associated with the rs174675 recessive C COMT polymorphism (p = 0.018), indicating that people without the rs174675 recessive C COMT polymorphism had 82% less chance of presenting joint disorder. Also, the feeling of never being as happy as the they could be (fourth item on the SHS) was associated with joint disorder (p = 0.037), so for each point higher on the SHS, the risk of TMJ increased by 37%.
Discussion
The etiology of TMD is multifactorial, involving physical, psychological,5 and genetic factors.4,12 The present study evaluated associations between TMJ, feelings of happiness, as well as polymorphisms in the COMT, HTR2A, and FKBP5 genes in Brazilian adolescents. As TMD is a complex condition with many diagnoses and different etiologies, we chose to study one group (TMJ) in order to be more specific and accurate about the possible associated aspects. As far as we know, this is the first study to evaluate the association between these factors and TMJ. The role of psychological and genetic factors in the etiology of TMJ requires further clarification.4,12 We hypothesized that emotional aspects and polymorphisms in the COMT, HTR2A, and FKBP5 genes contribute to the etiology of TMJ, as these factors can affect functions that may influence the condition.
Adolescence is a time of numerous changes.8 Thus, the development of mental problems, such as anxiety, unhappiness, and depression, is not uncommon in this period.9 A recent study found an association between TMD and depression,32 which draws attention to the influence of emotional factors. Another study showed that oral conditions can decrease the level of happiness in Brazilian adolescents.33 Happiness is a construct similar to the subjective feeling of well-being and is considered an important factor in psychology as a complementary construct of health mental. Thus, the investigation of feelings of happiness constitutes a subjective assessment of whether a person is happy or unhappy.29 In the present study, we found a significant association between happiness and TMJ, as adolescents who reported never being as happy as they could be (fourth item on Subjective Happiness Scale) were more likely to have TMJ. To the best of our knowledge, this is the first study to assess this issue and it may contribute to future discussions regarding the impact of subjective feelings of happiness on the etiology of TMJ and the emergence of pain related to this disorder.
The main findings of this study were the associations of TMJ with feelings of happiness and the associations of TMJ and polymorphisms in the COMT gene in adolescents. This gene is an important candidate for the etiology of painful conditions,17 as it regulates the expression of the enzyme catechol-O-methyltransferase, which is responsible for metabolizing the active catecholamine derivative and promoting the inactivation of acetylcholine.15,15Polymorphisms in the COMT gene are responsible for the malfunction of this enzyme, resulting in low reuptake and increased levels of catecholamines34,35 as well as the stimulation of β adrenergic receptors responsible for pain sensitivity.36
A recent systematic review with meta-analysis found that polymorphisms in the COMT gene may be associated with TMD.4 In the present study, we found that the rs174675 polymorphism in the COMT gene was associated with TMJ, as the prevalence of the disorder was higher among adolescents with this polymorphism. As far as we know, this polymorphism has not previously been investigated for its association with TMJ and may be included with other candidate polymorphisms associated with this condition.4,12
Polymorphisms in the HTR2A gene were also investigated in the present study. This gene encodes the serotonin receptor 5-hydroxytryptamine, which is considered an essential component of the serotonergic system, as it involves physiological functions, such as memory, sleep, nociception, feeding, and reward.18,19 The modulation of the functioning and activity of HTR2A is poorly understood, but this gene is believed to play a pro-nociceptive role in spinal pain by modulating serotonergic activity, causing an increase in nociceptive transmission that results in hyperalgesia and increased levels of continuous (neuropathic) pain.37 As TMD is the main cause of chronic non-dental orofacial pain,2 we hypothesized that it is associated with polymorphisms in HTR2A. Freitas et al.18 evaluated this association in Brazilian adults and found that the 102T-C polymorphism was associated with TMD.18 We found no studies that evaluated HTR2A polymorphisms in Brazilian adolescents, which demonstrates the importance of the present investigation. However, polymorphisms in the HTR2A gene were not associated with TMJ in the adolescents in our study.
To the best of our knowledge, this is the first study to evaluate the association between polymorphisms in the FKBP5 gene and TMJ. We found only two previous studies that investigated the association of such polymorphisms with oral conditions. Reis et al.24found an association between the rs3800373 polymorphism in the FKBP5 gene and greater surgical discomfort associated with third molar surgery in women. Scariot et al.34 investigated the association between polymorphisms (rs1360780, rs3800373) in the FKBP5 gene and bruxism in children. We speculated that polymorphisms in the FKBP5 gene would be associated with TMJ in adolescents due to the emotional and psychological aspects that influence the etiology of TMD4,5 and the fact that polymorphisms in this gene have previously been associated with post-traumatic stress disorder,25 anxiety,23 depressive disorder,23,24 and surgical discomfort.24 However, TMJ was not associated with polymorphisms in the FKBP5 gene in the present study.
In this study, we did not evaluate the direct association between feelings of happiness and polymorphisms in the COMT, HTR2A, and FKBP5 genes. However, future studies should assess the connection between these factors since these genes are linked to regulatory functions in the central nervous system, such as responses to stress, emotional aspects such as anxiety and depression, and subjective aspects such as quality of life and oral health-related quality of life.24,40-45 Furthermore, future studies should include a larger sample of adolescents, with the measurement of more comprehensive emotional aspects, and the evaluation of other polymorphisms in the same genes and/or different candidate genes to better understand the etiology of TMJ in this population.
This study provides useful information on emotional and genetic aspects in the development of TMJ in adolescents, which is important, considering the limited research on this condition in this specific age group. Moreover, as gene expression depends on life experiences,17 gene-environment interactions, especially with regards to stress and psychological problems, are likely less common among younger individuals in comparison to adults and older people. We used international criteria for the diagnosis of TMD28,38,39 and the assessment of feelings of happiness.30,31 We also had excellent kappa agreement coefficients, which indicates the reliability of the data. However, caution should be used when interpreting the results, as the study was conducted with a convenience sample and we only measured one subgroup (TMJ) of the three main TMD types (myofascial pain, disc displacement, and TMJ). Moreover, psychological and emotional aspects of the adolescents that could influence their perception of happiness were not investigated.
Conclusion
The present study found an association between TMJ and happiness in Brazilian adolescents, as adolescents who considered themselves less happy were more likely to have TMJ compared to those who considered themselves happier. The diagnosis of TMJ was also associated with a polymorphism in the COMT gene (rs174675), as homozygous C participants were more likely to have TMJ than heterozygous CT individuals.
Acknowledgment
This study was funded by Universidade Federal de Minas Gerais (UFMG - Federal University of Minas Gerais) and the following Brazilian fostering agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Coordination for the Advancement of Higher Education Personnel) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ - National Council of Scientific and Technological Development).
References
-
1 Herken H, Erdal E, Mutlu N, Barlas O, Cataloluk O, Oz F, et al. Possible association of temporomandibular joint pain and dysfunction with a polymorphism in the serotonin transporter gene. Am J Orthod Dentofacial Orthop. 2001 Sep;120(3):308-13. https://doi.org/10.1067/mod.2001.115307
» https://doi.org/10.1067/mod.2001.115307 -
2 List T, Jensen RH. Temporomandibular disorders: old ideas and new concepts. Cephalalgia. 2017 Jun;37(7):692-704. https://doi.org/10.1177/0333102416686302
» https://doi.org/10.1177/0333102416686302 -
3 Mladenovic I, Supic G, Kozomara R, Dodic S, Ivkovic N, Milicevic B, et al. Genetic polymor-phisms of catechol-o-methyltransferase: association with temporo-mandibular disorders and postoperative pain. J Oral Facial Pain Headache. 2016;30(4):302-10. https://doi.org/10.11607/ofph.1688
» https://doi.org/10.11607/ofph.1688 -
4 Brancher JA, Bertoli FM, Michels B, Lopes-Faturri A, Pizzatto E, Losso EM, et al. Is catechol-O-methyltransferase gene associated with temporomandibular disorders? A systematic review and meta-analysis. Int J Paediatr Dent. 2021 Jan;31(1):152-63. https://doi.org/10.1111/ipd.12721
» https://doi.org/10.1111/ipd.12721 -
5 Martins RJ, Garcia AR, Garbin CA, Sundefeld ML. Associação entre classe econômica e estresse na ocorrência da disfunção temporomandibular. Rev Bras Epidemiol. 2007;10(2):215-22. https://doi.org/10.1590/S1415-790X2007000200009
» https://doi.org/10.1590/S1415-790X2007000200009 -
6 LeResche L, Mancl LA, Drangsholt MT, Huang G, Von Korff M. Predictors of onset of facial pain and temporomandibular disorders in early adolescence. Pain. 2007 Jun;129(3):269-78. https://doi.org/10.1016/j.pain.2006.10.012
» https://doi.org/10.1016/j.pain.2006.10.012 -
7 Velly AM, Look JO, Schiffman E, Lenton PA, Kang W, Messner RP, et al. The effect of fibromyalgia and widespread pain on the clinically significant temporomandibular muscle and joint pain disorders: a prospective 18-month cohort study. J Pain. 2010 Nov;11(11):1155-64. https://doi.org/10.1016/j.jpain.2010.02.009
» https://doi.org/10.1016/j.jpain.2010.02.009 -
8 Best O, Ban S. Adolescence: physical changes and neurological development. Br J Nurs. 2021 Mar;30(5):272-5. https://doi.org/10.12968/bjon.2021.30.5.272
» https://doi.org/10.12968/bjon.2021.30.5.272 -
9 Mundy LK, Canterford L, Moreno-Betancur M, Hoq M, Sawyer SM, Allen NB, et al. Social networking and symptoms of depression and anxiety in early adolescence. Depress Anxiety. 2021 May;38(5):563-70. https://doi.org/10.1002/da.23117
» https://doi.org/10.1002/da.23117 -
10 Slade GD, Sanders AE, Ohrbach R, Bair E, Maixner W, Greenspan JD, et al. COMT Diplotype amplifies effect of stress on risk of temporomandibular pain. J Dent Res. 2015 Sep;94(9):1187-95. https://doi.org/10.1177/0022034515595043
» https://doi.org/10.1177/0022034515595043 - 11 Melis M, Di Giosia M. The role of genetic factors in the etiology of temporomandibular disorders: a review. Cranio. 2016;7:1-9.
-
12 Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 2011 Nov;12(11 Suppl):T92-101. https://doi.org/10.1016/j.jpain.2011.08.005
» https://doi.org/10.1016/j.jpain.2011.08.005 -
13 Michelotti A, Liguori R, Toriello M, D'Antò V, Vitale D, Castaldo G, et al. Catechol-O-methyltransferase (COMT) gene polymorphisms as risk factor in temporomandibular disorders patients from Southern Italy. Clin J Pain. 2014 Feb;30(2):129-33. https://doi.org/10.1097/AJP.0b013e318287a358
» https://doi.org/10.1097/AJP.0b013e318287a358 -
14 Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, et al. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 2010 Apr;20(4):239-48. https://doi.org/10.1097/FPC.0b013e328337f9ab
» https://doi.org/10.1097/FPC.0b013e328337f9ab -
15 Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999 Dec;51(4):593-628. https://doi.org/10.1016/S0031-6997 (24)01423-6
» https://doi.org/10.1016/S0031-6997 (24)01423-6 -
16 Antypa N, Drago A, Serretti A. The role of COMT gene variants in depression: bridging neuropsychological, behavioral and clinical phenotypes. Neurosci Biobehav Rev. 2013 Sep;37(8):1597-610. https://doi.org/10.1016/j.neubiorev.2013.06.006
» https://doi.org/10.1016/j.neubiorev.2013.06.006 -
17 Brancher JA, Spada PP, Meger MN, Fatturri AL, Dalledone M, de Paiva Bertoli FM, et al. The association of genetic polymorphisms in serotonin transporter and catechol-O-methyltransferase on temporomandibular disorders and anxiety in adolescents. J Oral Rehabil. 2019 Jul;46(7):597-604. https://doi.org/10.1111/joor.12783
» https://doi.org/10.1111/joor.12783 -
18 Freitas LV, Lopes AC, Piatto VB, Maniglia JV. Association of temporomandibular dysfunction with the 102T-C polymorphism in the serotonin receptor gene in Brazilian patients. Arch Med Sci. 2013 Dec;9(6):1013-8. https://doi.org/10.5114/aoms.2013.39215
» https://doi.org/10.5114/aoms.2013.39215 -
19 Okamoto K, Imbe H, Tashiro A, Kimura A, Donishi T, Tamai Y, et al. The role of peripheral 5HT2A and 5HT1A receptors on the orofacial formalin test in rats with persistent temporomandibular joint inflammation. Neuroscience. 2005;130(2):465-74. https://doi.org/10.1016/j.neuroscience.2004.10.004
» https://doi.org/10.1016/j.neuroscience.2004.10.004 -
20 Abe Y, Suganuma T, Ishii M, Yamamoto G, Gunji T, Clark GT, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res. 2012 Jun;21(3):289-96. https://doi.org/10.1111/j.1365-2869.2011.00961.x
» https://doi.org/10.1111/j.1365-2869.2011.00961.x -
21 Wu W, Li Z, Tang T, Wu J, Liu F, Gu L. 5-HTR2A and IL-6 polymorphisms and obstructive sleep apnea-hypopnea syndrome. Biomed Rep. 2016 Feb;4(2):203-8. https://doi.org/10.3892/br.2015.558
» https://doi.org/10.3892/br.2015.558 -
22 Mattina GF, Samaan Z, Hall GB, Steiner M. The association of HTR2A polymorphisms with obsessive-compulsive disorder and its subtypes: a meta-analysis. J Affect Disord. 2020 Oct;275:278-89. https://doi.org/10.1016/j.jad.2020.06.016
» https://doi.org/10.1016/j.jad.2020.06.016 -
23 Scheuer S, Ising M, Uhr M, Otto Y, von Klitzing K, Klein AM. FKBP5 polymorphisms moderate the influence of adverse life events on the risk of anxiety and depressive disorders in preschool children. J Psychiatr Res. 2016 Jan;72:30-6. https://doi.org/10.1016/j.jpsychires.2015.10.009
» https://doi.org/10.1016/j.jpsychires.2015.10.009 -
24 Reis GE, Calixto RD, Petinati MF, Souza JF, Kuchler EC, Costa DJ, et al. Effect of different factors on patient perception of surgical discomfort in third molar surgery. Braz Oral Res. 2020 Nov;35:e007. https://doi.org/10.1590/1807-3107bor-2021.vol35.0007
» https://doi.org/10.1590/1807-3107bor-2021.vol35.0007 -
25 Wang Q, Shelton RC, Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. J Affect Disord. 2018 Jan;225:422-8. https://doi.org/10.1016/j.jad.2017.08.066
» https://doi.org/10.1016/j.jad.2017.08.066 -
26 Farsi NM. Symptoms and signs of temporomandibular disorders and oral parafunctions among Saudi children. J Oral Rehabil. 2003 Dec;30(12):1200-8. https://doi.org/10.1111/j.1365-2842.2003.01187.x
» https://doi.org/10.1111/j.1365-2842.2003.01187.x -
27 Hongxing L, Astrøm AN, List T, Nilsson IM, Johansson A. Prevalence of temporomandibular disorder pain in Chinese adolescents compared to an age-matched Swedish population. J Oral Rehabil. 2016 Apr;43(4):241-8. https://doi.org/10.1111/joor.12366
» https://doi.org/10.1111/joor.12366 -
28 Franco-Micheloni AL, Fernandes G, Gonçalves DA, Camparis CM. Temporomandibular disorders among Brazilian adolescents: reliability and validity of a screening questionnaire. J Appl Oral Sci. 2014;22(4):314-22. https://doi.org/10.1590/1678-775720130694
» https://doi.org/10.1590/1678-775720130694 - 29 Pais-Ribeiro JL. Validação transcultural da escala de felicidade subjectiva de Lyubomirsky e Lepper. Psicol Saude Doencas. 2012;13(2):157-68.
- 30 Medeiros ED, do Nascimento AM, Mariano TE, Sales HFS, de Medeiros PCB. Escala de Felicidade de Lima: validade fatorial e consistência interna. Rev Psicol Pesquisa. 2014;8(2):150-8.
-
31 Küchler EC, Tannure PN, Falagan-Lotsch P, Lopes TS, Granjeiro JM, Amorim LM. Buccal cells DNA extraction to obtain high quality human genomic DNA suitable for polymorphism genotyping by PCR-RFLP and Real-Time PCR. J Appl Oral Sci. 2012;20(4):467-71. https://doi.org/10.1590/S1678-77572012000400013
» https://doi.org/10.1590/S1678-77572012000400013 -
32 Simoen L, Van den Berghe L, Jacquet W, Marks L. Depression and anxiety levels in patients with temporomandibular disorders: comparison with the general population. Clin Oral Investig. 2020 Nov;24(11):3939-45. https://doi.org/10.1007/s00784-020-03260-1
» https://doi.org/10.1007/s00784-020-03260-1 -
33 Tuchtenhagen S, Ortiz FR, Ardenghi TM, Antunes JL. Oral health and happiness in adolescents: a cohort study. Community Dent Oral Epidemiol. 2021 Apr;49(2):176-85. https://doi.org/10.1111/cdoe.12589
» https://doi.org/10.1111/cdoe.12589 - 34 Scariot R, Brunet L, Olsson B, Palinkas M, Regalo SC, Rebellato NL, et al. Single nucleotide polymorphisms in dopamine receptor D2 are associated with bruxism and its circadian phenotypes in children. Cranio. 2022 Mar;40(2):152-9.
-
35 Gong L, He C, Yin Y, Ye Q, Bai F, Yuan Y, et al. Nonlinear modulation of interacting between COMT and depression on brain function. Eur Psychiatry. 2017 Sep;45:6-13. https://doi.org/10.1016/j.eurpsy.2017.05.024
» https://doi.org/10.1016/j.eurpsy.2017.05.024 -
36 Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007 Apr;128(3):199-208. https://doi.org/10.1016/j.pain.2006.09.022
» https://doi.org/10.1016/j.pain.2006.09.022 -
37 Sachau J, Bruckmueller H, Gierthmühlen J, Magerl W, May D, Binder A, et al. The serotonin receptor 2A (HTR2A) rs6313 variant is associated with higher ongoing pain and signs of central sensitization in neuropathic pain patients. Eur J Pain. 2021 Mar;25(3):595-611. https://doi.org/10.1002/ejp.1696
» https://doi.org/10.1002/ejp.1696 -
38 Franco AL, Fernandes G, Gonçalves DA, Bonafé FS, Camparis CM. Headache associated with temporomandibular disorders among young Brazilian adolescents. Clin J Pain. 2014 Apr;30(4):340-5. https://doi.org/10.1097/AJP.0b013e31829ca62f
» https://doi.org/10.1097/AJP.0b013e31829ca62f -
39 Bertoli FM, Bruzamolin CD, Pizzatto E, Losso EM, Brancher JA, de Souza JF. Prevalence of diagnosed temporomandibular disorders: A cross-sectional study in Brazilian adolescents. PLoS One. 2018 Feb;13(2):e0192254. https://doi.org/10.1371/journal.pone.0192254
» https://doi.org/10.1371/journal.pone.0192254 -
40 Teixeira EC, das Neves BM, Castilho T, Silva Ramos T, Flaviana A, Carelli J, et al. Evidence of Association between MTRR and TNF-a Gene Polymorphisms and Oral Health-Related Quality of Life in Children with Anterior Open Bite. J Clin Pediatr Dent. 2022 May;46(3):249-58. https://doi.org/10.17796/1053-4625-46.3.12
» https://doi.org/10.17796/1053-4625-46.3.12 -
41 Antunes LA, Pinheiro LH, Castilho T, Todoroff N, Duarte C, Tavares JD, et al. Genetic polymorphisms in TNF-a as a potential biomarker for oral health-related quality of life in children. Braz Oral Res. 2022 May;36:e059. https://doi.org/10.1590/1807-3107bor-2022.vol36.0059
» https://doi.org/10.1590/1807-3107bor-2022.vol36.0059 -
42 Baldiotti AL, Amaral-Freitas G, Barbosa MC, Moreira PR, Machado RA, Coletta RD, et al. Associations between anxiety, depression, chronic pain and oral health-related quality of life, happiness, and polymorphisms in adolescents' genes. Int J Environ Res Public Health. 2023 Feb;20(4):3321. https://doi.org/10.3390/ijerph20043321
» https://doi.org/10.3390/ijerph20043321 -
43 Von Held R, Castilho T, Antunes LA, Tavares JD, Pivetta Petinati MF, Winckler C, et al. Interleukin 1 alpha genetic polymorphisms as potential biomarkers for oral health-related quality of life in Para athletes. Spec Care Dentist. 2021 Nov;41(6):679-87. https://doi.org/10.1111/scd.12627
» https://doi.org/10.1111/scd.12627 -
44 Sebastiani AM, Dos Santos KM, Cavalcante RC, Pivetta Petinati MF, Signorini L, Antunes LA, et al. Depression, temporomandibular disorders, and genetic polymorphisms in IL6 impact on oral health-related quality of life in patients requiring orthognathic surgery. Qual Life Res. 2020 Dec;29(12):3315-23. https://doi.org/10.1007/s11136-020-02581-8
» https://doi.org/10.1007/s11136-020-02581-8 -
45 Gabardo M, Zielak J, Tórtora G, Gerber J, Meger M, Rebellato N, et al. Impact of orthognathic surgery on quality of life: predisposing clinical and genetic factors. J Craniomaxillofac Surg. 2019 Aug;47(8):1285-91. https://doi.org/10.1016/j.jcms.2019.05.001
» https://doi.org/10.1016/j.jcms.2019.05.001

 Temporomandibular joint disorders, happiness, and COMT, HTR2A and FKBP5 polymorphisms in adolescents: a cross-sectional study
Temporomandibular joint disorders, happiness, and COMT, HTR2A and FKBP5 polymorphisms in adolescents: a cross-sectional study