Abstract
Plants have evolved elaborate signaling networks, believed to be necessitated by the diversity and complexity of their morphology, developmental and reproductive strategies, and the need to cope with an ever-changing environment from which they are rooted and cannot escape. Their receptor-like kinase superfamilies, with members numbering in the hundreds to more than a thousand, exemplify how plants have evolved their signaling versatility. FERONIA (FER) receptor kinase from model Arabidopsis is a member of the Malectin-domain receptor kinase family conserved among plants. FER has a perplexingly broad functional range, impacting growth to reproduction throughout the plant life cycle, and survival when encountering biotic and abiotic stressors from the environment, such as pathogens and climatic adversity. Efforts to understand FER signaling have brought to light novel signaling strategies at the continuum of the plant cell wall and plasma membrane, and a network of cytoplasmic and nuclear pathways that together support its biological roles. The discussion here focuses on the cell surface mechanisms, including a sugar-peptide interaction-driven liquid-liquid phase separation process along the cell wall-plasma membrane interface and a plasma membrane-linked signaling node comprised of FER, a glycosylphosphatidylinositol-anchored protein, the RHO GTPase molecular switch and a generator for reactive oxygen species (ROS). The emerging recognition of how the broader FER-related receptor kinase family could impact plant wellness and agricultural productivity is also discussed.
Keywords: Arabidopsis, GPI-AP, Rapid Alkalinization Factor, RHO GTPase, pectin, Liquid-liquid phase separation
Introduction
Studies of plant receptor kinases came into prominence starting from the mid-1980s, with the identification in the agriculturally important crop Zea mays (corn) and vegetable Brassica plants of receptor-like proteins that shared semblance with the epidermal growth factor family of receptor tyrosine kinases [1,2]. The genomic landscapes for plant receptor-like kinases (RLKs) were beginning to unfold starting from the early 2000s, establishing the presence of expansive arrays of these receptor proteins, eg, > 600 in the model plant Arabidopsis to > 1000 in Oryza sativa (rice) [3,4]. Categorized into more than 40 subfamilies, a predominant RLK group comprises transmembrane proteins that structurally resemble the animal receptor tyrosine kinases [5] in bearing an extracellular domain for signal perception, a transmembrane domain, and a cytoplasmic kinase domain, albeit they are predominantly serine/threonine kinases. The majority of these RLKs have leucine-rich repeats in their extracellular domains, and many are of central importance for plant growth, reproduction, immunity signaling, and stress management [6]. Examples include the Brassinosteroid-Insensitive 1 (BRI1) for perception of the steroid growth hormone brassinosteroide [7], the S-Locus receptor kinase [2] for self-incompatible male-female interaction, and Flagellin 22 sensing 2 (FLS2) for sensing bacterial pathogens [8]. In the decades since these discoveries, studies of plant receptor kinases have delineated numerous signaling pathways crucial for plant growth and survival. Mechanistic studies show that plant receptor kinases have evolved intricate mechanisms featuring both divergence and conservation in perception and intracellular signal relay pathways, including the prominent use of mitogen-activated protein kinase cascades and ubiquitination to mediate the signaling outcome [9-12]. Unabated efforts continue to push at the frontier of mechanistic understanding in the model plant Arabidopsis, and increasingly sophisticated gene editing efforts are expended by a global agricultural research community to study receptor kinases in crop species. Among the most actively researched are the Malectin-domain receptor kinases [13-16]. One of its founding members FERONIA (FER) (Figure 1) [17-21] is the subject of this discussion.
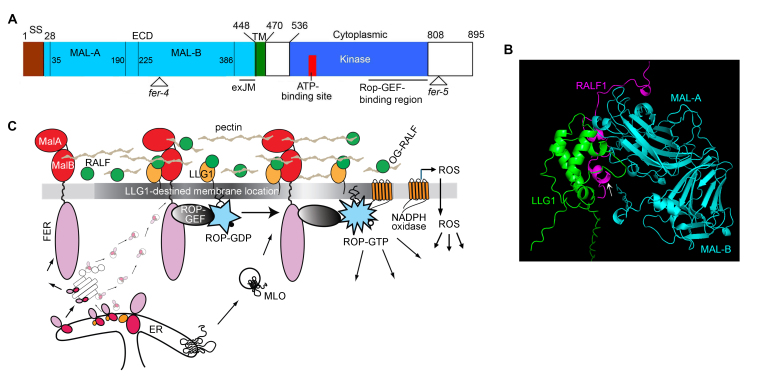
Figure 1.
FER receptor kinase. (A) Domain structure of FER. MALA,B are Malectin-like domains. exJM interacts with LLG1. Of the multiple T-DNA insertion and single site mutant fer alleles reported, fer-4 is the most studied knockout allele; fer-5 is an often-studied weaker T-DNA insertion allele affecting mostly root hair phenotypes. The C-terminal region interacts with RopGEF. (B) Alphafold3-generated model of full length RALF1-LLG1-FER extracellular domain. Model mimics the determined structure of FER-LLG2-RALF231-17 [67]. Arrow points to the RALF1 N-terminal short helical region that engages in interacting with LLG1. (C) The FER-LLG1 core signaling pathway. The “piggy-back” model [40] of LLG1-chaperoned transport of FER from the ER to LLG1-destined locations in the plasma membrane is shown. MLO7 translocation to the plasma membrane depends on FER and pollen tube arrival [78]. ROP-GTP and ROS are ubiquitous signaling molecules regulating diverse processes.
FER and the Malectin-domain Receptor Kinase Family
FER receptor kinase is a member of the CrRLK1-like family of the Arabidopsis RLK superfamily [3], after the founding member identified from the medicinal and ornamental plant Catharanthus roseus (Madagascar periwinkle). FER is one of the 17 members of the CrRLK1-like family and was first discovered as having a major role in female fertility in Arabidopsis, thus the namesake of the Etruscan goddess Feronia, associated with fertility, health, and abundance [22]. Identification of the FER gene coincided with the discovery of the animal protein Malectin, a di-glucose-binding protein in the endoplasmic reticulum lumen (ER) and functions in the early steps of the secretory pathway [23,24]. Increasingly, “Malectin-like” is used to reflect the presence of dual malectin-like regions in their extracellular domains (Figure 1A) [13-16]. A broader family of Malectin domain-containing receptor kinases are conserved across the plant kingdom.
Since its identification, FER has emerged as functionally the most far-reaching among plant receptor kinases, with the exceptional capacity to profoundly impact diverse processes throughout the plant life cycle, controlling growth, ensuring reproductive success, mediating plant wellness and resilience under normal and stressful growth conditions [17-21]. FER is broadly expressed, its functional contributions appear to predominate those from its co-expressing homologs because knockout mutations in FER alone adequately induce unambiguous, often severe, phenotypes. fer knock-out mutants are highly pleiotropic, revealing defects in growth controlled by growth hormones, pathogen- and other environmental stress-induced responses [25-31]. FER interacts directly with regulators of several major pathways, such as the red-light photoreceptor phytochrome, and functions in cell wall sensing, plant-soil microbiome interaction to facilitate plant wellness, Target of Rapamycin signaling and its connection to autophagy [32-36]. We refer readers to recent reviews covering the elaborate and intertwining cytoplasmic and nuclear signaling network associated with FER [17,18,21,37,38].
Here, we focus on the FER mechanisms in signal perception at the cell surface (the cell wall- plasma membrane interface) and signal dispatch intracellularly along the cytoplasmic surface of the plasma membrane to enable the functional versatility of FER. These mechanisms also resonate with fundamental signaling principles in other eukaryotic systems.
The FER Core Signaling Module at the Cell Wall-plasma Membrane Interface with Diverse Signaling Potential
The core FER signaling module refers to a dynamic molecular complex that comprises the transmembrane FER in association with a glycosylphosphatidylinositol-anchored protein (GPI-AP) as a coreceptor pair, their ligand (a secreted small peptide hormone called Rapid Alkalinization Factors (RALFs)), an inner membrane-linked molecular switch ROP (RHO GTPase of plants), and its activator guanine nucleotide exchange factor (ROP-GEF) and NADPH oxidase, a transmembrane ROP effector that generates reactive oxygen species (ROS) (Figure 1C) [17,18,39,40]. We discuss how this core module harnesses considerable capacity for functional diversification, contributing to the biological versatility of FER. To facilitate a discussion of FER, which plays major roles in mediating male-female interactions to enable reproductive success [18,41,42], we provide Box 1 for an overview of the specialized structures and processes involved as a roadmap.
Box 1.
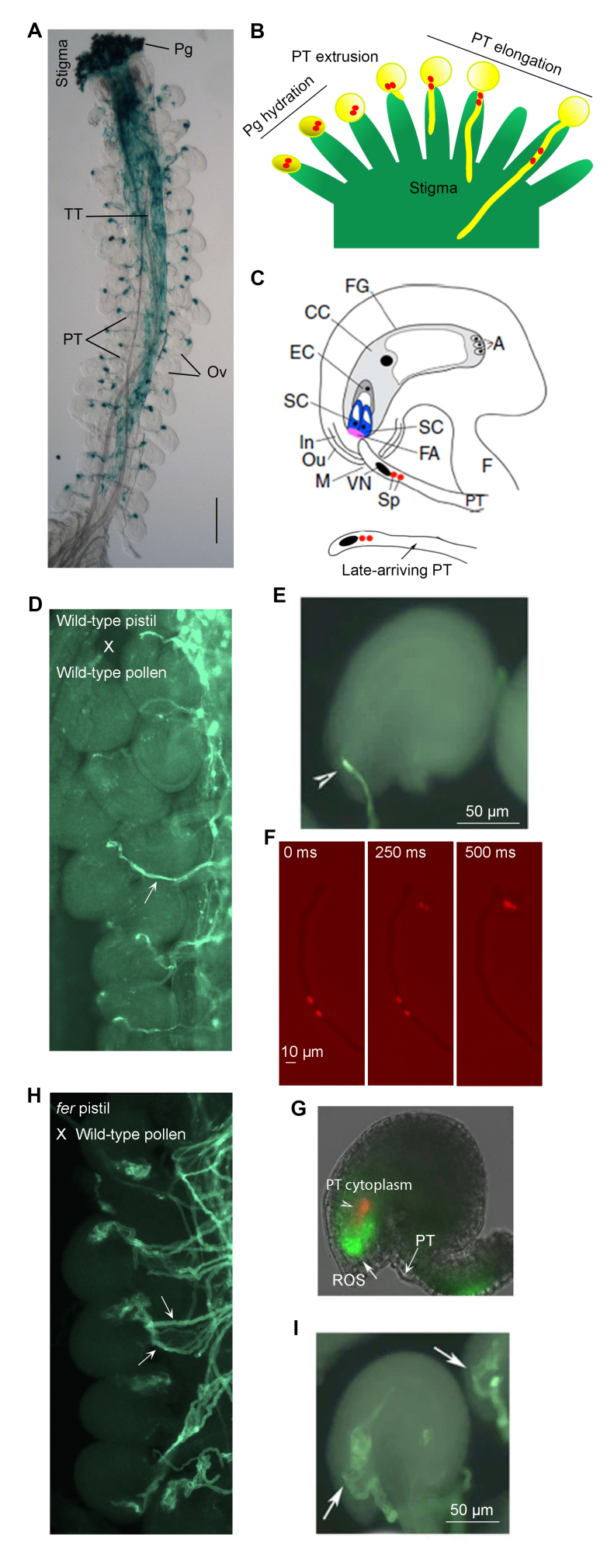
Prezygotic male-female interaction in flowering plant reproduction and the roles of FER. A pollinated Arabidopsis pistil showing the entire pollen tube (PT) journey is shown in (A). The stigma is the receptive surface for pollen grains (pg), seen here in blue from a transgene-expressed reporter. Pgs hydrate and germinate, each extruding a PT that penetrates the stigma as shown in (B). PTs then grow in the extracellular matrix secreted by the transmitting tissue (TT), they sense attractants from the ovules (Ov), and each exits the TT one at a time to target an Ov. Typically, an Ov is only penetrated by a single PT (C–E); late-arriving ones are deterred from approaching an already penetrated Ov (C). The pollen tube transports two sperm cells (red dots in B) in its cytoplasm until it enters an Ov as depicted in (B, C) and penetrates the female gametophyte (FG) where the egg cell (EC) resides. Two synergid cells (SC) are located at the entrance to the FG. They secrete a thickened cell wall region known as the filiform apparatus (FA). A dense network of SC membrane innervates the FA, concentrating FER and LRE there. The sperm cells (sp) and the PT nucleus (oval body inside the tube) are maintained just distal to the tube tip. Upon penetrating one of the two SCs, the PT burst immediately, releasing the two sperm cells. (D) shows PTs (arrow) exiting the TT one at a time, ending the journey at an Ov. (E) shows a wild-type Ov with a PT, stained with a cell wall dye, having entered the FG and ruptured (so terminating growth, arrowhead). (F) shows a PT undergoing rupture and sperm release in vitro in conditions similar to that conferred by FER. The rupture is abrupt as seen in these images captured at 250 msec intervals showing release is complete within 0.5 sec. (G) shows an Ov with a ROS maximum at the entrance to the FG, a condition conferred by FER, with the released PT cytoplasm (in red). (H) shows loss-of-FER mutant (fer-4) Ov are approached by multiple PTs (arrows), which continue to grow inside the FG but do not rupture, giving rise to the PT pile up phenotype (I). The fertilized FG and Ov give rise to the female embryo and seed, respectively. (C) also shows: In, Ou (inner and outer integuments) develop into the future seed coat.; M, micropyle, the aperture through which the PT gains access to the FG; CC, central cell, the second female gamete, fertilized by the 2nd sperm cell and develops into the endosperm (storage tissue) of the future seed; A, antipodal cells, together with the CC, EC and SCs, they make up the 7-celled FG, typical of many flowering plants. Some panels are reproduced from [42,49,85]; others are comparable results shown in these papers.
A Receptor Kinase-GPI-AP Coreceptor Pair
FER partners with a GPI-AP from the LRE-like GPI-AP (LLG)/LORELEI (LRE) protein family as a co-receptor [39,40]. FER is expressed almost ubiquitously except in pollen grains, the specialized sperm-bearing male cells, where FER homologs are present. The four members of the LLG/LRE protein family are differentially expressed [43]. LLG1 is expressed in vegetative tissues, including in the stigma where pollen grains are deposited (Box 1). LRE is expressed in the paired synergid cells that gate the female gametophyte, located inside the ovule, where fertilization takes place. LLG2 and LLG3 are exclusively expressed in the pollen; they partner with FER counterparts in pollen grains to ensure pollen tube arrival at the ovule for fertilization [44,45]. The expression of LLG1 and LRE together spans the expression spectrum of FER. The biological activity of these LLG/LRE are interchangeable [43]. The FER-LLG1/LRE partnership was discovered because phenotypes of loss-of-LLG1 and those of loss-of-LRE mutants span the full spectrum of knockout fer mutants during growth and reproduction, respectively [40]. fer and knock-out llg1 plants are highly pleiotropic during growth and defective in the female ability to maintain mating barriers in the stigma (Box 1), to prevent penetration by unwanted pollen from incompatible plants [46-48]. Fertilization occurs in germ cells inside the female gametophyte (Box 1), and is blocked in knockout fer and lre mutants, severely suppressing female fertility. FER and LRE, acting as coreceptors, are both required to create an environment that induces sperm release from the penetrating pollen tube [49] (Box 1). Several recent reviews have discussed these FER-LLG1/LRE control phenomena comprehensively [13,18,50].
GPI-APs as Chaperones Delivering FER to its Functional Location in the Plasma Membrane
GPI-AP proteins are secreted to the cell surface where many remain linked to the outer leaflet of the plasma membrane via its GPI-anchor. They are conserved through protozoa to animals and plants, playing important roles in a wide range of processes, including development, immunity, and interacting with regulators to orchestrate signaling from the cell surface [51-57]. Much is still to be elucidated on how GPI-APs execute these functions. They exit the ER-Golgi in vesicles already destined for localization in sterol and sphingolipid-enriched subdomains of the plasma membrane. Referred to as “lipid-rafts” or micro/nanodomains [58-63] and segregated from the bulk-phase membrane, these sterol and sphingolipid-enriched membrane subdomains are specialized for orchestrating signaling from the cell surface. FER and LLG1 interact in the ER with LLG1 binding to an extracellular juxta membrane (exJM) region of FER (Figure 1A) [40]. However, in the llg1 knock-out mutants, while FER is still notably present in the plasma membrane, much of it is trapped in the ER. Clearly, the plasma membrane-located FER, presumably secreted there via the default secretory pathway, is inactive, thus resulting in llg1 phenocopying fer during the vegetative growth phase. Therefore, these results suggest that only FER complexed with LLG1 is active, leading to a model whereby the pool of LLG1-bound FER is chaperoned by LLG1 by “piggybacking” onto the GPI-AP, and together they exit the ER and are trafficked to the Golgi, then LLG1-destined locations in the plasma membrane (Figure 1C). LRE apparently also functions similarly in the ovules [40].
Interaction with LLG1/LRE is clearly a bottleneck that needs to be passed to ascertain the biological roles of FER are precisely executed despite being broadly and quite abundantly expressed. Partnering with differentially expressed homologs expands the functional spectrum without engaging more elaborate regulatory mechanisms. Plasma membrane-anchored GPI-APs lack a cytoplasmic domain and, therefore, have no cytoplasmic signaling capacity. Providing a trafficking route for a receptor kinase to capacitate its signaling activity is therefore a mechanistic strategy whereby a GPI-AP exerts its impact on signaling. Given that GPI-APs are predominantly located in membrane micro/nanodomains where many regulatory molecules are believed to congregate [51-57], FER, being chaperoned to where LLG1 is located, would be in a micro-environment concentrated with regulators of diverse processes, facilitating their interactions and therefore enable FER’s extraordinarily broad biological roles.
RALF1-FER-LLG1: A Prototypical Ligand-coreceptor Complex for FER Family Receptor Kinases
RALFs are secreted peptides discovered in the early 1980s and are noted for their growth inhibitory activities and conservation across plant species [64,65]. RALF1 were identified as a ligand of FER. It triggered phosphorylation of FER and of the growth regulatory H+-ATPase AHA2, and displayed FER-dependent root growth inhibition activity [66]. RALF23 was shortly determined to also bind FER to regulate FLS2-mediated immunity signaling [26]. In the tertiary ligand-coreceptor structure, an N-terminal 17-amino acid fragment from RALF23 interacts directly with the pollen-expressed LLG2, which closely mimics LLG1 in structural and biological interactions [43,67] (see Figure 1B).
We shall use RALF1-FER-LLG1 here as prototypical of the broader FER-related signaling modules. RALF1 and a majority of the RALFs are produced as pre-pro-peptides, and the mature peptides are secreted to the apoplast (the intercellular space between plant cells occupied by the cell wall matrix and freely diffusing molecules) [64,65]. Typically, Arabidopsis RALF expression is not highly specific and multiple RALFs are co-expressed to various levels in the same cell and tissue types [44,65]. Multiple RALFs could target the same coreceptor pairs, ensuring RALF availability to FER allowing it to function across the spectrum of its broad expression profile. Mature RALF1 has 59 amino acid residues, including two pairs of cysteine residues. Except for a short N-terminal region, which assumes an α-helix (Figure 1B) and includes several amino acid residues that are conserved and required for its activity to alkalinize the extracellular space, the rest of RALF1 is intrinsically disordered [64-67]. These properties are shared by most RALFs. The propensity of intrinsically disordered regions to engage in multivalent interactions [68] contributes to how RALF1 participates in FER-LLG1 signaling [69].
FER-LLG1-ROP to ROS Signaling Pathway: a Coreceptor – RHO GTPase-NADPH Oxidase Signaling Module
ROPs are a family of small GTPases closely related to animal Rac/Rho GTPases and major signaling hubs for many distinct extracellular signals and control diverse cytoplasmic pathways using a variety of effectors [70,71]. Their activity is regulated by GEFs, referred to as ROP-GEFs, switching them from the GDP-bound inactive form to the GTP-bound activated form. In a search of upstream regulators of ROPs, FER was found to bind ROP-GEF directly and functions as a cell surface receptor to activate ROPs, which in turn recruit NADPH oxidase and stimulate ROS production (Figure 1C) [72].
The FER-LLG1 to ROS pathway operates during the vegetative growth phase to support normal seedling development, controlling polarized root hair growth and cell shape morphogenesis to generate a jigsaw puzzle-patterned leaf epidermis [40,49,72-74]. During the reproductive phase, several female tissue-expressed RALFs function with FER-LLG1 to maintain ROP-mediated ROS production as a barrier at the stigma (Box 1) [46,75]. The stigmatic gate responds to compatible pollen, which downregulates the FER-LLG1-controlled pathway and relaxes the ROS-maintained barrier, allowing pollen hydration to extrude a pollen tube for penetrating the pistil. The FER-regulated pathway is stimulated by incompatible pollen, elevating the ROS environment to arrest the unwanted mates, preventing their invasion into the pistil [46-48,75]. Distinct pollen-secreted peptide ligands from compatible and incompatible pollen grains interact with the stigmatic RALF1-FER-LLG1 module to render the stigmatic environment either hospital or hostile to the courting mates.
At the final stage of pollen invasion, the pollen tube arrives at the ovule to gain entry to where the female gametes are located within the female gametophyte (Box 1). FER-LRE mediates a ROS environment to induce explosive rupture of the penetrating pollen tube, ejecting its cytoplasm and two cargo sperm cells to enable fertilization [18,49] (Box 1). Although FER is not expressed in pollen or pollen tubes, signaling modules comprised of pollen counterparts of FER and LLG1/LRE also control ROS production in pollen tubes [44,45]. There is an interesting dichotomy here: contrary to the FER-LRE maintained ROS environment that acts exogenously to weaken the cell wall and induce rupture of the invading pollen to enable fertilization, the pollen FER-LLG1 related module regulated ROS production mediates pollen tube cell wall integrity to ensure its growth until they reach the female target to achieve fertilization [18,41]. Interestingly, the FER-mediated ROS even mediates inter-kingdom communication between plant roots and its soil microbiome and regulates the level of beneficial bacteria in the rhizosphere, which in turn impacts plant wellness [34].
ROPs are major molecular switches that regulate myriad signaling pathways [70,71]. ROS are ubiquitous regulators important from cell growth to cell death and are crucial for defense responses [76,77]. In connecting FER to downstream processes, a FER-LLG1 to ROP-regulated ROS production pathway provides immense potential for signal diversification.
Further Elaborations: Linkages to Ca2+ and Nitric Oxide
Ca2+, a universal second messenger, is intimately linked to RALF-FER-LLG1/LRE signaling. RALFs trigger transient Ca2+ influx [64-66]. In the ovule, FER-LRE interacts with a seven transmembrane protein belonging to the Powdery Mildew resistance Locus O family, MLO7 (Figure 1C) [78]. An approaching pollen tube induces notable changes in the cytoplasmic Ca2+ dynamics of the synergid cells at the entrance of the female gametophyte (Box 1). MLO7 is believed to be a Ca2+ channel contributing to this response [79], although MLOs recruiting a cyclic nucleotide gated Ca2+ channel has also been noted [80]. Furthermore, the pollen tube-induced, FER-LRE-MLO7-mediated Ca2+ response in the synergid cells most likely involves other yet-to-be-identified Ca2+ channels since the contribution from MLO7 to the Ca2+-influx dependent sperm release process [49] is substantially weaker than from FER-LRE, leading to the suggestion that MLO7 functions as a booster to FER-LRE [81].
Nitric Oxide (NO), a gaseous signaling molecule, regulates many physiological processes in animals and plants [82-84]. It serves as a crucial mediator for FER-regulated processes [18,42,48,85]. In the stigmatic pollen barrier (Box 1), compatible pollen rapidly triggers an increase in stigmatic NO, followed by the relaxation of the FER-maintained ROS gate, allowing pollen germination and tube entry. Reversely, incompatible pollen fails to trigger a NO response, and stigmatic FER-mediated ROS remains high, blocking pollen entry. Nitroso-modified FER and NADPH oxidase together suppress the FER to ROS pathway [48]. In the ovule, along with mediating sperm release to enable fertilization [49], FER-regulated NO blocks entry by late-arriving pollen tubes when fertilization by the first penetrating pollen tube is assured, thus averting polyspermy [85] (Box 1).
Pectin-FER Interaction: A Cell Wall to Plasma Membrane Continuum
The presence of dual Malectin-like domains in the extracellular domain of FER family of receptor kinases (Figure 1A) generated intense interest that they might interact with cell wall carbohydrates and function as sensors to transmit perturbations in the cell wall to signal cellular responses [86,87]. While providing integrity to the encased cell, the cell wall is also dynamic and capable of responding to extracellular conditions, such as physical damages, as well as intracellular demands, such as those imposed by cell expansion, division, and cell shape morphogenesis [88,89,90]. The cellulosic cell wall matrix is crisscrossed with pectin, which are highly and variably modified homogalacturonans and the most dynamic component of the cell wall matrix (see Box 2 for a summary of FER-pectin connection) [90]. De-esterified pectin interacts with FER and RALF1 [69,85,91], connecting the cell wall with the plasma membrane. Leucine-rich repeat extensins (LRXs) are a family of proteins that are tightly associated with the cell wall [92,93]. They have been implicated with a cell wall sensing role via interactions with FER- and FER-related receptor kinase-RALF signaling modules [94,95,96]. In vitro as well as in growing pollen tubes and root hairs, de-esterified pectin interacts with RALFs complexed to the Leucine-rich repeat region of several LRX [95,96]. Therefore, FER and FER related receptor-pectin-RALF-LRX also maintains a cell wall-plasma membrane continuum in the tip-growing cells.
Box 2.
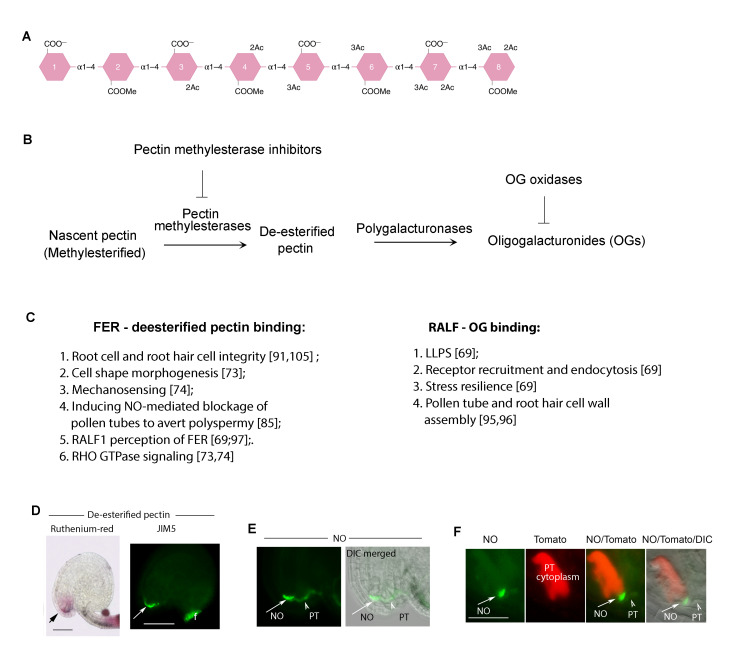
The pectin connection. (A) A sketch of pectin (homogalacturonan) with various modifications, including methylesterification (COOMe) and its de-esterified form (COO-). Reproduced from [90]. (B) Pectin de-esterification and hydrolysis to produce oligogalacturonides (OGs) and the enzymes involved. Pectin is secreted in methylesterified form. Cell wall located pectin methylesterases (PMEs) modify the nascent pectins, their exposed COO- will cross-link via Ca2+ bridges, rigidifying the wall; their activities are counteracted by PME Inhibiting (PMEI) enzymes in the wall. Together PME and PMEI maintain pectin homeostasis. Plant and microbial polygalacturonases hydrolyze de-esterified pectins. Small pectin fragments, oligalacturonides (OGs) are biologically active, which is inactivated by OG oxidases [99]. (C) Partial lists of the functional involvements by FER-de-esterified pectin interaction and RALF-de-esterified pectin interaction. See Supplementary Movie 1 for salt inducing swelling in wild type root cells which remain intact (upper) and also swelling in fer-4 roots, which rupture reflecting their compromised cell wall integrity. (D-F) FER-de-esterified pectin induced NO-mediated pollen tube blockade to prevent polyspermy. (D) FER-pectin interaction maintains de-esterified pectin (stained by Ruthenimum red, or antibody JIM5) accumulation at the entrance to the female gametophyte. (E) FER-maintained pectin and pollen tube (pt) arrival induce NO accumulation at the entrance to the female gametophyte. (F) shows NO marked female gametophyte entrance and the ejected pollen tube cytoplasm (in red).
Pectin
Pectin dynamics are intimately linked to RALF1-FER-LLG1 signaling [69,97] (Box 2). Two forms of pectin, methylesterified and de-esterified, mediate cell wall malleability and stiffness oppositely, rendering these polysaccharides powerful agents in regulating its dynamics. Pectin is secreted as methylesterified homogalacturonan. A battery of pectin restructuring enzymes, such as various pectinases that reduce its degree of polymerization (DP), are in the plant cell wall [90,98]. Pectin methylesterases and Pectin methylesterase inhibitors act counteractively to maintain distributions between methylesterifed and de-esterified pectins as required by the status of the cell. Methylesterified pectin is loosely associated with the wall matrix; it mediates wall malleability, accommodating cell expansion and rendering penetrability to invading agents, such as pollen tubes that grow within the cell wall matrix of female tissues (Box 1). De-esterified pectin is variably associated with the cell wall matrix. In response to cationic conditions, such as [Ca2+] in the wall matrix, de-esterified pectin crosslinks with each other via Ca2+ bridges between their free carboxyl groups, rigidifying the cell wall and rendering it more able to withstand cell turgor, thus maintaining cell integrity. It also renders the cell wall more protective of penetrations by invading agents such as pathogens. Pathogens secrete cell wall degradative enzymes, in particular various pectinases, to degrade the host cell walls to gain penetrance. Oligogalacturonans (OGs), pectin fragmented to different DP, eg, between 10-15 (OGDP10-15), are biologically active [99-103]. They function as elicitors to trigger host cell defense, such as turning on defense-related genes and closing stomata, pores on leaf surfaces for gaseous exchange, but are also used as entry points by pathogens. Biologically active OGs are inactivated by OG oxidases [98]. Cellular conditions such as various growth hormones could trigger cell wall modifications that could feedback regulate cellular activities needed for processes such as cell expansion, differentiation, and division [104,105]. Pectin methylesterase, de-esterified pectin, and OG have all been implicated as crucial for cell wall-related phenomena mediated by RALF-FER-LLG1 [69,97].
Pectin-FER Interaction Impacts Cell Wall Quality and Sensing
De-esterified pectin binds to both MALA and MALB from the FER extracellular domain (Figure 1A,B) [69,73,74,91]. The presence of FER is linked to the stable accumulation of de-esterified pectin on the cell surface, such as in the walls of specialized female reproductive tissues [85] (see Box 2). FER-pectin interaction mediates cell wall strength, which is important for cell growth and reproduction and protects it from stress-induced wall damage [40,91,105]. During vegetative growth, FER-pectin interaction is important for the morphogenesis of the jigsaw puzzle pattern of the leaf epidermis [40,73]. Cell elongation imposes stress on the cell wall, FER-pectin interaction maintains cell wall integrity during the growth hormone brassinosteroid-induced rapid growth phase [105]. When encountering stress, eg, high salinity, FER-pectin interaction prevents root cells from bursting and mediates resilience for survival [69,91]. Loss-of-FER resulted in fer mutant seedlings not being able to regain growth even when the stress conditions were mitigated. FER-pectin interaction is therefore important to maintain cell wall integrity, presumably by stabilizing de-esterified pectin associated with the wall matrix.
The FER-mediated and NO-executed polyspermy block in the ovules referred to earlier is also dependent on a FER-mediated, synergid cell-secreted, de-esterified pectin-enriched cell wall at the entrance to the female gametophyte (see Box 1 and Box 2) [85]. Growing pollen tubes secrete pectinases, producing pectic fragments that stimulate NO accumulation at the entrance to the female gametophyte, coincident with the arrival of the first pollen tube. Loss of FER diminished the pectin accumulation and rendered NO undetectable. Late-arriving pollen tubes continued to enter mutant ovules that had already received a pollen tube, giving rise to multiple pollen tubes gaining entries. This, together with pollen tubes failing to rupture due to the diminished ROS environment [49], gives rise to the dramatic pile-up of pollen tubes inside the female gametophyte that fer mutants are noted for (Box 1).
The NO-mediated blockade functions at least on two levels [85]. First, the FER- and de-esterified pectin-dependent NO accumulation at the entrance of the penetrated female gametophyte nitrosates LUREs and inactivates these secreted pollen tube attractants, and also prevents further LURE secretion, thus disengaging the mechanism that would guide more pollen tubes to an already penetrated ovule. Secondly, rupture of the already penetrated pollen tube generates an abrupt increase in [Ca2+] at the female gametophyte entrance. Though it remains to be directly demonstrated, the abrupt increase in [Ca2+] would induce extensive crosslinking between the de-esterified pectin, stiffening the cell wall matrix to ensure its non-penetrability by any late-arriving pollen tubes. Together, the FER to ROS plasma membrane to cytoplasmic signaling pathway and the cell wall-associated FER-pectin-NO pathway act to enable fertilization and avert polyspermy, respectively, thus ensuring maximum seed production and healthy progenies [18,42].
OG-RALF1 Liquid-Liquid Phase Separation (LLPS) at the Cell Surface Mediates FER-LLG1-Dependent Resilience to Environmental Stress and Enables its Almost Global Functionality
RALF1-FER-LLG1 interaction at the plant cell surface bears characteristics exceptional to typical ligand-receptor interaction [69]. Efforts to understand the molecular underpinning of these biological phenomena led to the discovery of RALF1 interaction with de-esterified pectin, RALF1-OG LLPS, and FER and LLG1 recruitment into RALF1-OG condensates in vitro and in vivo, and how the in vivo process impacts plant growth resilience [69].
RALF1 Triggers FER- and LLG1-dependent Promiscuous Endocytosis
Ligand-receptor interaction typically triggers downstream signaling specifically mediated by the cognate receptor. This is generally followed by the endocytosis of the ligand-receptor complex to modulate intracellular signaling activities [106,107]. RALF1, however, induces not only FER and LLG1 endocytosis but also that of a battery of non-cognate targets in the plasma membrane in a FER- and LLG1-dependent manner [69]. Among these non-cognate targets are the cell growth-regulating H+-ATPase, several receptor kinases such as the receptor kinases BRI1 and FLS2 for the growth hormone brassinosteroid and bacterial elicitor flagellin 22, influx and efflux transporters PIN and AUX of the hormone auxin, respectively. Growth and the activity of H+-ATPases, brassinosteroid, auxin, and immunity signaling are intimately linked to FER-LLG1 functions [17,19,21]. RALF1 also augments uptake of the endocytic marker dye FM4-64, implying that the impact from signal activation of FER-LLG1 could be even more widespread. Loss of FER or LLG1 obliterates these RALF1-induced responses, revealing FER-LLG1 as a mediator of RALF1-induced promiscuous endocytosis. These results reflect that in response to signal activation, FER-LLG1 could simultaneously regulate the activity of numerous cellular pathways for diverse processes thus mediating a cell surface mechanism that enables its exceptionally broad functional range.
Pectin-RALF LLPS In Vitro
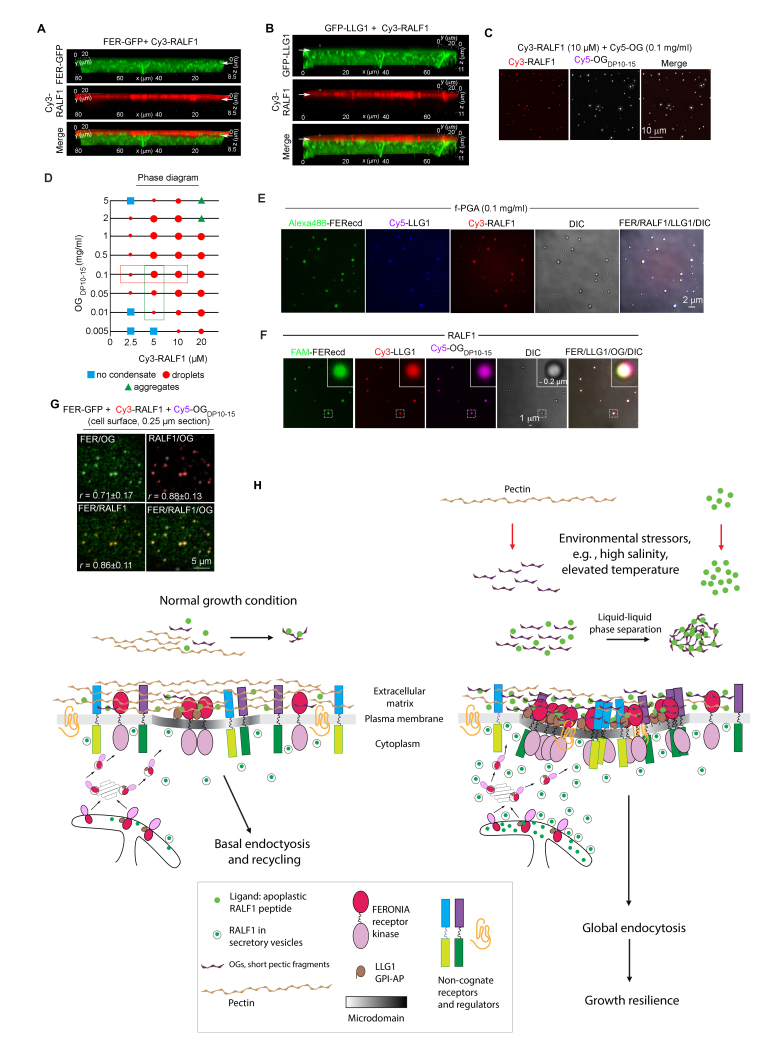
As a process that drives biomolecules to concentrate into condensates more readily able to overcome thresholds for their reactivities, the importance of LLPS in biological processes has long been recognized [108-112]. De-esterified pectin fragments are anionic (Box 2) and therefore should readily interact with cationic molecules in its milieu; pectin is known to undergo phase transition in the cell wall [113]. Besides being disordered rendering it prone for phase separation [114], RALFs are highly basic, eg, the pI of RALF1 is around 10.5, with 12 of its 49 amino acid residues being positively charged [69]. OG of various sizes, in particular OGDP10-15 and OGDP25-50, interact with several RALF peptides in vitro [69,95,96]. In the case of RALF1, when mixed with OG, they readily formed condensates with molecular dynamics and biochemical properties aligned with being products of LLPS (Figure 2C,D). FER extracellular domain and LLG1 are readily recruited to OG-RALF1, forming condensates of OG-RALF1-FER-LLG1 (Figure 2E,F).
Figure 2.
OG-RALF1 LLPS in vitro and on the cell surface. (A,B) Cy3-RALF1-induced FER-GFP (A) and GFP-LLG1 (B) endocytosis, but RALF1 remains extracellular in the cell wall. Arrows indicate the cell wall-plasma membrane interface. See Supplementary Movies 2,3 for RALF1-induced receptor clustering and endocytosis. (C) Condensates of Cy3-RALF1 and Cy5-OG, shown in single channels and merged Cy3/Cy5. (D) Phase diagram summarizing OG-RALF1 dose-response relationships. (E,F) FER extracellular domain (ecd), LLG1 assembly with OG-RALF1. (F) RALF1 is labeled (Cy3); fragmented polygalacturonic acid (fPGA, DP > 50) is not. (G) RALF1 is unlabeled, OGDP10-15 is labeled (Cy5). (G) OG-RALF1-FER receptor clusters on the cell surface. (H) A model for RALF1-OG LLPS triggered promiscuous receptor clustering and endocytosis. Left panel: Under ambient growth conditions, a basal level of FER-LLG1 receptor uptake and recycling occurs. Right panel: Under stressful conditions, apoplastic levels of RALFs and OG are elevated, conditions leading to RALF-OG LLPS and promiscuous receptor recruitment and endocytosis, thus simultaneously modulating numerous cell surface regulated pathways to protect from excessive stress-induced damages and allow for resilience to develop. (Reproduced from [69]).
A panel of RALF1 mutants altered specifically with eliminated key positively charged amino acid residues, cysteine and tyrosine residues speculated to contribute to LLPS, were tested for their efficacy in binding to OG. None devastated the capacity, but all produced significantly fewer and smaller peptide-OG condensates than those formed with wild-type RALF1. These observations, therefore, revealed parameters that contribute to OG-RALF1 interaction are consistent with those typical of disordered protein regions.
OG-RALF1 Phase Separation at the Cell Surface Mediates Receptor Recruitment and Endocytosis
Experiments using differentially labeled OG, RALF1, FER, and LLG1 established that the RALF1-triggered receptor clusters comprise all four of these components (Figure 2G). Similar to in vitro assembly of these receptor clusters (Figure 2E,F), both OG and RALF1 are required in RALF1-induced receptor clusters and endocytosis in vivo [69]. Fragmented pectin produced on the cell surface, typically de-esterified, or applied OGs are incorporated into the RALF1-triggered receptor clusters. Chemically or genetically reducing the level of de-esterified pectin inhibited these processes. RALF1-OG interaction also recruits other non-cognate targets, such as the immunity and brassinosteroid receptors FLS2 and BRI1, respectively, to co-cluster with FER, revealing a molecular basis of how RALF1 triggers the endocytosis of these non-cognate receptors. The RALF1 condensates are dynamic and sensitive to hexanediol, a diagnostic for LLPS due to its disruption of weak hydrophobic interaction resulting in condensate dissolution. Mutant RALF1s that displayed compromised capacity to interact with OG and in OG-RALF1 condensate formation in vitro also had reduced efficacy than wild-type RALF1 in inducing receptor endocytosis and inhibiting root growth. Together, these results are consistent with RALF1-triggered receptor clusters and endocytosis being mediated by LLPS.
Environmental Stress Triggers OG-RALF1 Phase Separation and Induces FER- and LLG1-Dependent Promiscuous Endocytosis as a Coping Mechanism
Succumbing to environmental stressors such as heat and drought devastates plant growth and productivity. Stressors like heat and drought attenuate plant growth, a coping response protecting plants from excessive growth-induced damage, so they are better able to emerge from growth arrest and regain growth when more favorable conditions return [115,116]. FER-LLG1 is important for plant survival from biotic and abiotic stressors and does so, at least partially, by mediating stress-induced promiscuous endocytosis [69]. It was determined that environmental stressors such as elevated temperature or high salinity stimulate mature RALF secretion and increase the production of fragmented pectin in the extracellular matrix. This is akin to applying RALF peptides exogenously or experimentally augmenting the level of de-esterified pectin to drive pectin-RALF-FER-LLG1 receptor clustering and endocytosis at the cell surface (see Supplementary Movie 1,2). Under stress, RALF1-OG phase separates and assembles into surface condensates, recruiting FER, LLG1, and other receptors, driving their clustering and elevating their overall endocytosis. Loss of FER, LLG1, or genetically reducing the plant capacity to secrete RALFs or produce de-esterified pectin were all inhibitory to heat- and salt-stress-induced promiscuous endocytosis. These mutant plants also lost their stress resilience and failed to emerge from growth arrest even when ambient conditions were restored. Therefore, the stress-induced, pectin-RALF-FER-LLG1-mediated cell surface response could attenuate numerous cell-surface regulator-regulated pathways, preventing irreversible damages, thus facilitating a coping mechanism that could recruit the participation of many signaling pathways for diverse processes.
FER-LLG1 as Anchors for Membrane Microdomain Assembly: A Global Signaling Strategy?
As discussed earlier, LLG1 binds FER and chaperones the receptor kinase to its functional location [40]. The GPI-AP-occupied sterol- and sphingolipid-enriched membrane subdomains [51-63] are known to be where signaling molecules, such as receptors, tend to concentrate and initiate their signaling activity. Segregation of sterols and sphingolipids from bulk-phase membrane lipids is a classic example of LLPS. RALF1-FER-LLG1 interaction induces numerous other cell surface regulators to coalesce with the FER-LLG1 coreceptor clusters could be triggered by the formation of micro/nano-domains nucleated by the peptide ligand binding to sterol- and sphingolipid-located LLG1. Among the plasma membrane- associated proteins that are endocytosed upon RALF1 treatment are REMORINs, proteins that have been shown biochemically to be associated with sterol- and sphingolipid membrane fractions and microscopically observed to reside in membrane micro/nanodomains [63]. Associated with the cytoplasmic surface of the plasma membrane, REMORINs have been proposed to serve as scaffolds for the assembly of other microdomain constituents. Therefore, while requiring further analyses, the assembly of OG-RALF-FER-LLG1 could be part of a broader mechanism that nucleates microdomains assembly.
Beginning from the ER, FER-LLG1 is a partnership of intriguing design. Lacking a cytoplasmic domain for signal dispatch, LLG1 nevertheless not only serves a critical role for FER functional capacitation but also could more broadly mark the membrane subdomains that it occupies for the assembly of other cell surface signaling molecules. The recruitment of many regulatory molecules for diverse processes into microdomain environments provides proximity to FER-LLG1. This might also underlie observations of FER cytoplasmic domain interacting with a large array of cytoplasmic molecules, some with key biological functions [30,38,115,116].
Beyond RALF1-FER-LLG1 and Model Arabidopsis
Given that RALF1, FER, and LLG1 are all members of multiple protein families and that they are conserved across the plant kingdom. Combinatorically, they harness tremendous signaling potential. We discuss briefly here some RALF1-FER-LLG1 related modules from Arabidopsis that have been studied extensively and the expanding efforts to study these signaling molecules from many crop species.
RALF1-FER-LLG1 Related Modules in Arabidopsis
The RALF1-FER-LLG1 related ligand-coreceptor modules in Arabidopsis studied thus far suggest the constituent proteins provide similar biochemical and molecular capacities to play different roles in the context of where they are expressed. For example, the leaf-abundant RALF23-FER-LLG1 plays an important role in regulating immunity responses to pathogens [26]. At least two other FER-related receptor kinases, LETUM1 and LETUM2, also anchor modules with important roles in disease signaling. LLG1 binds both LETUM1 and LETUM2 [117,118] to regulate a downstream MAPK cascade with the LETUM1/2-LLG1 complex serving as a scaffold for the assembly with additional components of the immunity signaling pathway. The male and female organs and cells have their designated constituents, such as the FER-LRE coreceptor assembled at the entrance of the female gametophyte for female fertility (Box 1) and the BUPS1/2-ANXUR1/2-LLG2/3-RALF4/19 modules in pollen [18,44,45,49]. Loss of any of the components of these pollen modules results in male sterility [44,45]. ANXURs and BUPSs form obligatory dimers; loss of function of both ANXURs, or loss of function in BUPSs induces male sterility. Several FER-related receptor kinases are also expressed in the stigma and ovules, and combinatorial mutations in these receptors induce subsets of fer phenotypes [46,75,119]. While initially recognized as pollen-specific, ANXURs are also expressed during vegetative growth in developmental stages vulnerable to diseases. ANXUR1 and ANXUR2 together play an important regulatory role in immunity signaling, linking cell surface pattern recognition receptors to intracellular receptors for pathogen effectors [120]. Moreover, mutational studies uncoupled ANXUR immunity and reproduction functions, revealing the evolution of distinct molecular interactions to serve different biological roles. These examples reflect not only how RALF-FER-LLG1 signaling can be broadly influential but also reveal intriguing designs that expand the biological roles of individual malectin-domain receptor kinases.
RALF1-FER-LLG1 Related Modules in Agriculturally Important Plant Species
Studies of FER signaling in Arabidopsis have led to efforts in achieving translationally important goals, such as overcoming interspecific reproduction barriers, which carries important implications for species diversification and increased productivity resulting from hybrid-rigor. Loss of FER, FER-related receptor kinases, stigma-expressed RALFs resulted in relaxing the pollination barriers for several distantly related interspecific species, allowing penetration of the pollen tubes [48,75]. Moreover, in the vegetable crop Brassica rapa, genetically down-regulating the stigmatic RALF-FER-LLG1-controlled ROS production pathway rendered its stigma more readily penetrable by pollen from distant species, resulting in hybrid embryo formation [48]. This opens the door to generating hybrids from species with various desirable traits, such as pathogen resistance, into the cultivated Brassica species.
To-date, FER-related signaling modules have been implicated in a broad range of agricultural species, revealing potential roles in mediating resistance to pathogens and environment stresses in major staple crops like maize and rice to vegetable, fruit and industrially important crops such as tomato, apples, and cotton [121-130]. RALF homologs have also been identified in animal and microbial disease-causing agents, such as nematodes and Fusarium [131-134]. As more advances are uncovered in how the RAFL-FER-LLG1 signaling module contributes to the performance of agricultural species, the knowledge should contribute to rational designs to improve crop yields, ascertaining production and food security to meet global nutritional needs.
Supplementary Material
Salt-induced root cell responses. Top, a wild-type root; Bottom, a fer-4 root.
RALF1 induced FER-GFP clustering and endocytosis.
RALF1 induced GFP-LLG1 clustering and endocytosis.
Acknowledgments
Works from the authors’ laboratory were supported by NSF/IOS:1127002, 1146941, 1645858, 2101467; NSF/MCB-1715764, NIH R01GM147548, and NIFA/USDA, the University of Massachusetts Center for Agriculture, Food, and the Environment (MAS00525).
Glossary
- FER
FERONIA
- RLKs
receptor-like kinases
- ER
endoplasmic reticulum
- BRI1
Brassinosteroid-Insensitive 1
- FLS-2
Flagellin 22 sensing 2
- GPI-AP
glycosylphosphatidylinositol-anchored protein
- RALFs
Rapid Alkalinization Factors
Conflict of Interest
Authors declare no conflict of interest.
Author Contributions
Both authors contributed to the conceptualization and writing of the manuscript.
References
- Walker JC, Zhang R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature. 1990. Jun;345(6277):743–6. 10.1038/345743a0 [DOI] [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA. 1991. Oct;88(19):8816–20. 10.1073/pnas.88.19.8816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003. Jun;132(2):530–43. 10.1104/pp.103.021964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004. May;16(5):1220–34. 10.1105/tpc.020834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010. Jun;141(7):1117–34. 10.1016/j.cell.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dievart A, Gottin C, Périn C, Ranwez V, Chantret N. Origin and Diversity of Plant Receptor-Like Kinases. Annu Rev Plant Biol. 2020. Apr;71(1):131–56. 10.1146/annurev-arplant-073019-025927 [DOI] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997. Sep;90(5):929–38. 10.1016/S0092-8674(00)80357-8 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 2006. Feb;18(2):465–76. 10.1105/tpc.105.036574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escocard de Azevedo Manhães AM, Ortiz-Morea FA, He P, Shan L. Plant plasma membrane-resident receptors: surveillance for infections and coordination for growth and development. J Integr Plant Biol. 2021. Jan;63(1):79–101. 10.1111/jipb.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Tang D, Wang W. The role of u iquitination in plant immunity: fine-tuning immune signaling and beyond. Plant Cell Physiol. 2022. Oct;63(10):1405–13. 10.1093/pcp/pcac105 [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang S. Mitogen-activated protein kinase cascades in plant signaling. J Integr Plant Biol. 2022. Feb;64(2):301–41. 10.1111/jipb.13215 [DOI] [PubMed] [Google Scholar]
- Herrmann A, Sepuru KM, Bai P, Endo H, Nakagawa A, Kusano S, et al. Chemical genetics reveals cross-regulation of plant developmental signaling by the immune peptide-receptor pathway. Sci Adv. 2025. Feb;11(6):eads3718. 10.1126/sciadv.ads3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck CM, Westermann J, Boisson-Dernier A. Plant Malectin-Like Receptor Kinases: From Cell Wall Integrity to Immunity and Beyond. Annu Rev Plant Biol. 2018. Apr;69(1):301–28. 10.1146/annurev-arplant-042817-040557 [DOI] [PubMed] [Google Scholar]
- Yang H, Wang D, Guo L, Pan H, Yvon R, Garman S, et al. Malectin/Malectin-like domain-containing proteins: A repertoire of cell surface molecules with broad functional potential. Cell Surf. 2021. Jun;7:100056. 10.1016/j.tcsw.2021.100056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Morea FA, Liu J, Shan L, He P. Malectin-like receptor kinases as protector deities in plant immunity. Nat Plants. 2022. Jan;8(1):27–37. 10.1038/s41477-021-01028-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblas VG, Gonneau M, Höfte H. Cell wall integrity signaling in plants: malectin-domain kinases and lessons from other kingdoms. Cell Surf. 2018. Jul;3:1–11. 10.1016/j.tcsw.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY. FERONIA: A Receptor Kinase at the Core of a Global Signaling Network. Annu Rev Plant Biol. 2024. Jul;75(1):345–75. 10.1146/annurev-arplant-102820-103424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Duan Q, Li C, James Liu MC, Wu HM. Pollen-pistil interactions: it takes two to tangle but a molecular cast of many to deliver. Curr Opin Plant Biol. 2022. Oct;69:102279. 10.1016/j.pbi.2022.102279 [DOI] [PubMed] [Google Scholar]
- Zhu S, Fu Q, Xu F, Zheng H, Yu F. New paradigms in cell adaptation: decades of discoveries on the CrRLK1L receptor kinase signalling network. New Phytol. 2021. Nov;232(3):1168–83. 10.1111/nph.17683 [DOI] [PubMed] [Google Scholar]
- Solis-Miranda J, Quinto C. The CrRLK1L subfamily: one of the keys to versatility in plants. Plant Physiol Biochem. 2021. Sep;166:88–102. 10.1016/j.plaphy.2021.05.028 [DOI] [PubMed] [Google Scholar]
- Xie Y, Sun P, Li Z, Zhang F, You C, Zhang Z. FERONIA receptor kinase integrates with hormone signaling to regulate plant growth, development and responses to environmental stimuli. Int J Mol Sci. 2022. Mar;23(7):3730. 10.3390/ijms23073730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007. Aug;317(5838):656–60. 10.1126/science.1143562 [DOI] [PubMed] [Google Scholar]
- Schallus T, Jaeckh C, Fehér K, Palma AS, Liu Y, Simpson JC, et al. Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol Biol Cell. 2008. Aug;19(8):3404–14. 10.1091/mbc.e08-04-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T, Fehér K, Sternberg U, Rybin V, Muhle-Goll C. Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology. 2010. Aug;20(8):1010–20. 10.1093/glycob/cwq059 [DOI] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant. 2010. May;3(3):626–40. 10.1093/mp/ssq015 [DOI] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science. 2017. Jan;355(6322):287–9. 10.1126/science.aal2541 [DOI] [PubMed] [Google Scholar]
- Zhao C, Zayed O, Yu Z, Jiang W, Zhu P, Hsu CC, et al. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2018. Dec;115(51):13123–8. 10.1073/pnas.1816991115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Nolan TM, Song G, Liu S, Xie Z, Chen J, et al. FERONIA Receptor Kinase Contributes to Plant Immunity by Suppressing Jasmonic Acid Signaling in Arabidopsis thaliana. Curr Biol. 2018. Oct;28(20):3316–3324.e6. 10.1016/j.cub.2018.07.078 [DOI] [PubMed] [Google Scholar]
- Yu F, Qian L, Nibau C, Duan Q, Kita D, Levasseur K, et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA. 2012. Sep;109(36):14693–8. 10.1073/pnas.1212547109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Park CH, Hsu CC, Kim YW, Ko YW, Zhang Z, et al. Mapping the signaling network of BIN2 kinase using TurboID-mediated biotin labeling and phosphoproteomics. Plant Cell. 2023. Mar;35(3):975–93. 10.1093/plcell/koad013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Park JS, Park HB, Moon KB, Kim HS, Jeon JH, et al. FERONIA Confers Resistance to Photooxidative Stress in Arabidopsis. Front Plant Sci. 2021. Jul;12:714938. 10.3389/fpls.2021.714938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang W, Li Y, Nie H, Cui L, Li R, et al. FERONIA coordinates plant growth and salt tolerance via the phosphorylation of phyB. Nat Plants. 2023. Apr;9(4):645–60. 10.1038/s41477-023-01390-4 [DOI] [PubMed] [Google Scholar]
- Liu C, Yu H, Voxeur A, Rao X, Dixon RA. FERONIA and wall-associated kinases coordinate defense induced by lignin modification in plant cell walls. Sci Adv. 2023. Mar;9(10):eadf7714. 10.1126/sciadv.adf7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wilson AJ, Zhang XC, Thoms D, Sohrabi R, Song S, et al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat Plants. 2021. May;7(5):644–54. 10.1038/s41477-021-00914-0 [DOI] [PubMed] [Google Scholar]
- Pacheco JM, Song L, Kuběnová L, Ovečka M, Berdion Gabarain V, Peralta JM, et al. Cell surface receptor kinase FERONIA linked to nutrient sensor TORC signaling controls root hair growth at low temperature linked to low nitrate in Arabidopsis thaliana. New Phytol. 2023. Apr;238(1):169–85. 10.1111/nph.18723 [DOI] [PubMed] [Google Scholar]
- Wang P, Clark NM, Nolan TM, Song G, Whitham OG, Liao CY, et al. FERONIA functions through Target of Rapamycin (TOR) to negatively regulate autophagy. Front Plant Sci. 2022. Aug;13:961096. 10.3389/fpls.2022.961096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Xu G, Li T, Zhou H, Lin Q, Chen J, et al. The RALF1-FERONIA complex interacts with and activates TOR signaling in response to low nutrients. Mol Plant. 2022. Jul;15(7):1120–36. 10.1016/j.molp.2022.05.004 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhu S, Ming Z, Liu X, Yu F. FERONIA cytoplasmic domain: node of varied signal outputs. aBIOTECH. 2020. Mar;1(2):135–46. 10.1007/s42994-020-00017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wu HM, Cheung AY. FERONIA and her pals: functions and mechanisms. Plant Physiol. 2016. Aug;171(4):2379–92. 10.1104/pp.16.00667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yeh FL, Cheung AY, Duan Q, Kita D, Liu MC, et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015. Jun;4:e06587. 10.7554/eLife.06587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Cheung AY, Qu LJ. Pollen tube integrity regulation in flowering plants: insights from molecular assemblies on the pollen tube surface. New Phytol. 2019. Apr;222(2):687–93. 10.1111/nph.15645 [DOI] [PubMed] [Google Scholar]
- Zhong S, Zhao P, Peng X, Li HJ, Duan Q, Cheung AY. From gametes to zygote: mechanistic advances and emerging possibilities in plant reproduction. Plant Physiol. 2024. Apr;195(1):4–35. 10.1093/plphys/kiae125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JA, Bielski NV, Liu MJ, DeFalco TA, Stegmann M, Nelson AD, et al. Evolutionary analysis of the LORELEI gene family in plants reveals regulatory subfunctionalization. Plant Physiol. 2022. Nov;190(4):2539–56. 10.1093/plphys/kiac444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science. 2017. Dec;358(6370):1596–600. 10.1126/science.aao3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Zhao Y, Liu MC, Zhou LZ, Wang L, Zhong S, et al. LLG2/3 Are Co-receptors in BUPS/ANX-RALF Signaling to Regulate Arabidopsis Pollen Tube Integrity. Curr Biol. 2019. Oct;29(19):3256–3265.e5. 10.1016/j.cub.2019.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Shen L, Xiao Y, Vyshedsky D, Peng C, Sun X, et al. Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science. 2021. Apr;372(6538):171–5. 10.1126/science.abc6107 [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang J, Su S, Wei X, Yang L, Zhao H, et al. FERONIA receptor kinase-regulated reactive oxygen species mediate self-incompatibility in Brassica rapa. Curr Biol. 2021. Jul;31(14):3004–3016.e4. 10.1016/j.cub.2021.04.060 [DOI] [PubMed] [Google Scholar]
- Huang J, Yang L, Yang L, Wu X, Cui X, Zhang L, et al. Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature. 2023. Feb;614(7947):303–8. 10.1038/s41586-022-05640-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun. 2014;5(1):3129. 10.1038/ncomms4129 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Harper JF, Palanivelu R. A fruitful journey: pollen tube navigation from germination to fertilization. Annu Rev Plant Biol. 2019. Apr;70(1):809–37. 10.1146/annurev-arplant-050718-100133 [DOI] [PubMed] [Google Scholar]
- Zurzolo C, Simons K. Glycosylphosphatidylinositol-anchored proteins: membrane organization and transport. Biochim Biophys Acta. 2016. Apr;1858(4):632–9. 10.1016/j.bbamem.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Maeda Y, Fujita M. Transport of glycosylphosphatidylinositol-anchored proteins from the endoplasmic reticulum. Biochim Biophys Acta. 2013. Nov;1833(11):2473–8. 10.1016/j.bbamcr.2013.01.027 [DOI] [PubMed] [Google Scholar]
- Paladino S, Lebreton S, Zurzolo C. Trafficking and Membrane Organization of GPI-Anchored Proteins in Health and Diseases. Curr Top Membr. 2015;75:269–303. 10.1016/bs.ctm.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Komura N, Suzuki KG, Ando H, Konishi M, Koikeda M, Imamura A, et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat Chem Biol. 2016. Jun;12(6):402–10. 10.1038/nchembio.2059 [DOI] [PubMed] [Google Scholar]
- Kinoshita T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol. 2020. Mar;10(3):190290. 10.1098/rsob.190290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KG, Kusumi A. Refinement of Singer-Nicolson fluid-mosaic model by microscopy imaging: lipid rafts and actin-induced membrane compartmentalization. Biochim Biophys Acta Biomembr. 2023. Feb;1865(2):184093. 10.1016/j.bbamem.2022.184093 [DOI] [PubMed] [Google Scholar]
- Guo Z, Kundu S. Recent research progress in glycosylphosphatidylinositol-anchored protein biosynthesis, chemical/chemoenzymatic synthesis, and interaction with the cell membrane. Curr Opin Chem Biol. 2024. Feb;78:102421. 10.1016/j.cbpa.2023.102421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997. Jun;387(6633):569–72. 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- Goñi FM. “Rafts”: A nickname for putative transient nanodomains. Chem Phys Lipids. 2019. Jan;218:34–9. 10.1016/j.chemphyslip.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Ott T. Membrane nanodomains and microdomains in plant-microbe interactions. Curr Opin Plant Biol. 2017. Dec;40:82–8. 10.1016/j.pbi.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Ott T. The Nanoscale Organization of the Plasma Membrane and Its Importance in Signaling: A Proteolipid Perspective. Plant Physiol. 2020. Apr;182(4):1682–96. 10.1104/pp.19.01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Bayer E, Bergmann DC, Botella MA, Boutté Y, Bozkurt TO, et al. Guidelines for naming and studying plasma membrane domains in plants. Nat Plants. 2024. Aug;10(8):1172–83. 10.1038/s41477-024-01742-8 [DOI] [PubMed] [Google Scholar]
- Jarsch IK, Ott T. Perspectives on remorin proteins, membrane rafts, and their role during plant-microbe interactions. Mol Plant Microbe Interact. 2011. Jan;24(1):7–12. 10.1094/MPMI-07-10-0166 [DOI] [PubMed] [Google Scholar]
- Blackburn MR, Haruta M, Moura DS. Twenty Years of Progress in Physiological and Biochemical Investigation of RALF Peptides. Plant Physiol. 2020. Apr;182(4):1657–66. 10.1104/pp.19.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abarca A, Franck CM, Zipfel C. Family-wide evaluation of RAPID ALKALINIZATION FACTOR peptides. Plant Physiol. 2021. Oct;187(2):996–1010. 10.1093/plphys/kiab308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014. Jan;343(6169):408–11. 10.1126/science.1244454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Stegmann M, Han Z, DeFalco TA, Parys K, Xu L, et al. Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature. 2019. Aug;572(7768):270–4. 10.1038/s41586-019-1409-7 [DOI] [PubMed] [Google Scholar]
- Orand T, Jensen MR. Binding mechanisms of intrinsically disordered proteins: insights from experimental studies and structural predictions. Curr Opin Struct Biol. 2025. Feb;90:102958. 10.1016/j.sbi.2024.102958 [DOI] [PubMed] [Google Scholar]
- Liu MJ, Yeh FJ, Yvon R, Simpson K, Jordan S, Chambers J, et al. Extracellular pectin-RALF phase separation mediates FERONIA global signaling function. Cell. 2024. Jan;187(2):312–330.e22. 10.1016/j.cell.2023.11.038 [DOI] [PubMed] [Google Scholar]
- Nibau C, Wu HM, Cheung AY. RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 2006. Jun;11(6):309–15. 10.1016/j.tplants.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Feiguelman G, Fu Y, Yalovsky S. ROP GTPases Structure-Function and Signaling Pathways. Plant Physiol. 2018. Jan;176(1):57–79. 10.1104/pp.17.01415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010. Oct;107(41):17821–6. 10.1073/pnas.1005366107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Tang W, Pan X, Huang A, Gao X, Anderson CT, et al. Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Curr Biol. 2022. Feb;32(3):497–507.e4. 10.1016/j.cub.2021.11.030 [DOI] [PubMed] [Google Scholar]
- Tang W, Lin W, Zhou X, Guo J, Dang X, Li B, et al. Mechano-transduction via the pectin-FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr Biol. 2022. Feb;32(3):508–517.e3. 10.1016/j.cub.2021.11.031 [DOI] [PubMed] [Google Scholar]
- Lan Z, Song Z, Wang Z, Li L, Liu Y, Zhi S, et al. Antagonistic RALF peptides control an intergeneric hybridization barrier on Brassicaceae stigmas. Cell. 2023. Oct;186(22):4773–4787.e12. 10.1016/j.cell.2023.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida AJ, de Oliveira JC, da Silva Pontes LV, de Souza Júnior JF, Gonçalves TA, Dantas SH, et al. ROS: basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxid Med Cell Longev. 2022. Oct;2022:1225578. 10.1155/2022/1225578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. ROS Are Good. Trends Plant Sci. 2017. Jan;22(1):11–9. 10.1016/j.tplants.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, et al. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010. Nov;330(6006):968–71. 10.1126/science.1195211 [DOI] [PubMed] [Google Scholar]
- Gao Q, Wang C, Xi Y, Shao Q, Li L, Luan S. A receptor-channel trio conducts Ca2+ signalling for pollen tube reception. Nature. 2022. Jul;607(7919):534–9. 10.1038/s41586-022-04923-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JG, Liang L, Jia PF, Wang YC, Li HJ, Yang WC. Integration of ovular signals and exocytosis of a Ca2+ channel by MLOs in pollen tube guidance. Nat Plants. 2020. Feb;6(2):143–53. 10.1038/s41477-020-0599-1 [DOI] [PubMed] [Google Scholar]
- Ju Y, Yuan J, Jones DS, Zhang W, Staiger CJ, Kessler SA. Polarized NORTIA accumulation in response to pollen tube arrival at synergids promotes fertilization. Dev Cell. 2021. Nov;56(21):2938–2951.e6. 10.1016/j.devcel.2021.09.026 [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E. Nitric oxide signaling in health and disease. Cell. 2022. Aug;185(16):2853–78. 10.1016/j.cell.2022.06.010 [DOI] [PubMed] [Google Scholar]
- Andrabi SM, Sharma NS, Karan A, Shahriar SM, Cordon B, Ma B, et al. Nitric oxide: physiological functions, delivery, and biomedical applications. Adv Sci (Weinh). 2023. Oct;10(30):e2303259. 10.1002/advs.202303259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos P, Prado AM, Wong A, Gehring C, Feijo JA. Nitric oxide: a multitasked signaling gas in plants. Mol Plant. 2015. Apr;8(4):506–20. 10.1016/j.molp.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Duan Q, Liu MJ, Kita D, Jordan SS, Yeh FJ, Yvon R, et al. FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature. 2020. Mar;579(7800):561–6. 10.1038/s41586-020-2106-2 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM. THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol. 2011. Dec;14(6):632–41. 10.1016/j.pbi.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Kessler SA, Grossniklaus U. The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J Exp Bot. 2011. Mar;62(5):1581–91. 10.1093/jxb/erq445 [DOI] [PubMed] [Google Scholar]
- De Lorenzo G, Ferrari S, Giovannoni M, Mattei B, Cervone F. Cell wall traits that influence plant development, immunity, and bioconversion. Plant J. 2019. Jan;97(1):134–47. 10.1111/tpj.14196 [DOI] [PubMed] [Google Scholar]
- Delmer D, Dixon RA, Keegstra K, Mohnen D. The plant cell wall-dynamic, strong, and adaptable-is a natural shapeshifter. Plant Cell. 2024. May;36(5):1257–311. 10.1093/plcell/koad325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Anderson CT, Xiao C. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat Plants. 2022. Apr;8(4):332–40. 10.1038/s41477-022-01120-2 [DOI] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr Biol. 2018. Mar;28(5):666–675.e5. 10.1016/j.cub.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borassi C, Sede AR, Mecchia MA, Salgado Salter JD, Marzol E, Muschietti JP, et al. An update on cell surface proteins containing extensin-motifs. J Exp Bot. 2016. Jan;67(2):477–87. 10.1093/jxb/erv455 [DOI] [PubMed] [Google Scholar]
- Herger A, Dünser K, Kleine-Vehn J, Ringli C. Leucine-Rich Repeat Extensin Proteins and Their Role in Cell Wall Sensing. Curr Biol. 2019. Sep;29(17):R851–8. 10.1016/j.cub.2019.07.039 [DOI] [PubMed] [Google Scholar]
- Dünser K, Gupta S, Herger A, Feraru MI, Ringli C, Kleine-Vehn J. Extracellular matrix sensing by FERONIA and Leucine-Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J. 2019. Apr;38(7):e100353. 10.15252/embj.2018100353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussu S, Lee HK, Haas KT, Broyart C, Rathgeb U, De Bellis D, et al. Plant cell wall patterning and expansion mediated by protein-peptide-polysaccharide interaction. Science. 2023. Nov;382(6671):719–25. 10.1126/science.adi4720 [DOI] [PubMed] [Google Scholar]
- Schoenaers S, Lee HK, Gonneau M, Faucher E, Levasseur T, Akary E, et al. Rapid alkalinization factor 22 has a structural and signalling role in root hair cell wall assembly. Nat Plants. 2024. Mar;10(3):494–511. 10.1038/s41477-024-01637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rößling AK, Dünser K, Liu C, Lauw S, Rodriguez-Franco M, Kalmbach L, et al. Pectin methylesterase activity is required for RALF1 peptide signalling output. eLife. 2024. Oct;13:RP96943. 10.7554/eLife.96943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yu Y, Liang Y, Anderson CT, Cao J. A Profusion of Molecular Scissors for Pectins: Classification, Expression, and Functions of Plant Polygalacturonases. Front Plant Sci. 2018. Aug;9:1208. 10.3389/fpls.2018.01208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Verrascina I, Pontiggia D, Locci F, Mattei B, De Lorenzo G, et al. Four Arabidopsis berberine bridge enzyme-like proteins are specific oxidases that inactivate the elicitor-active oligogalacturonides. Plant J. 2018. Apr;94(2):260–73. 10.1111/tpj.13852 [DOI] [PubMed] [Google Scholar]
- Voxeur A, Habrylo O, Guénin S, Miart F, Soulié MC, Rihouey C, et al. Oligogalacturonide production upon Arabidopsis thaliana-Botrytis cinerea interaction. Proc Natl Acad Sci USA. 2019. Sep;116(39):19743–52. 10.1073/pnas.1900317116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo IS, Oh KY, Choi EJ, et al. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999. Sep;121(1):147–52. 10.1104/pp.121.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Sun G, Yu Q, Gao T, Zhu Q, Wang R, et al. A plant mechanism of hijacking pathogen virulence factors to trigger innate immunity. Science. 2024. Feb;383(6684):732–9. 10.1126/science.adj9529 [DOI] [PubMed] [Google Scholar]
- Pontiggia D, Benedetti M, Costantini S, De Lorenzo G, Cervone F. Dampening the DAMPs: How Plants Maintain the Homeostasis of Cell Wall Molecular Patterns and Avoid Hyper-Immunity. Front Plant Sci. 2020. Dec;11:613259. 10.3389/fpls.2020.613259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Mravec J, Greiner S, Mouille G, Höfte H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol. 2012. Sep;22(18):1732–7. 10.1016/j.cub.2012.07.036 [DOI] [PubMed] [Google Scholar]
- Chaudhary A, Hsiao YC, Jessica Yeh FL, Župunski M, Zhang H, Aizezi Y, et al. FERONIA signaling maintains cell wall integrity during brassinosteroid-induced cell expansion in Arabidopsis. Mol Plant. 2025. Feb;•••:S1674–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013. May;5(5):a017459. 10.1101/cshperspect.a017459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani NG, Russinova E. Receptor endocytosis and signaling in plants. Curr Opin Plant Biol. 2009. Dec;12(6):653–9. 10.1016/j.pbi.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30(1):39–58. 10.1146/annurev-cellbio-100913-013325 [DOI] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017. May;18(5):285–98. 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Ditlev JA, Rosen MK. Regulation of Transmembrane Signaling by Phase Separation. Annu Rev Biophys. 2019. May;48(1):465–94. 10.1146/annurev-biophys-052118-115534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Velazquez CL, Dinneny JR. Organization out of disorder: liquid-liquid phase separation in plants. Curr Opin Plant Biol. 2018. Oct;45 Pt A:68–74. 10.1016/j.pbi.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Emenecker RJ, Holehouse AS, Strader LC. Biological Phase Separation and Biomolecular Condensates in Plants. Annu Rev Plant Biol. 2021. Jun;72(1):17–46. 10.1146/annurev-arplant-081720-015238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KT, Wightman R, Peaucelle A, Höfte H. The role of pectin phase separation in plant cell wall assembly and growth. Cell Surf. 2021. May;7:100054. 10.1016/j.tcsw.2021.100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang Y, Chen J. Backbone interactions and secondary structures in phase separation of disordered proteins. Biochem Soc Trans. 2024. Feb;52(1):319–29. 10.1042/BST20230618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu J, Gong Z, Zhu JK. Abiotic stress responses in plants. Nat Rev Genet. 2022. Feb;23(2):104–19. 10.1038/s41576-021-00413-0 [DOI] [PubMed] [Google Scholar]
- Zhao C, Jiang W, Zayed O, Liu X, Tang K, Nie W, et al. The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl Sci Rev. 2020. Jun;8(1):nwaa149. 10.1093/nsr/nwaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Huang Y, Kong L, Yu X, Feng B, Liu D, et al. The malectin-like receptor-like kinase LETUM1 modulates NLR protein SUMM2 activation via MEKK2 scaffolding. Nat Plants. 2020. Sep;6(9):1106–15. 10.1038/s41477-020-0748-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yin C, Liu J, Feng B, Ge D, Kong L, et al. A trimeric CrRLK1L-LLG1 complex genetically modulates SUMM2-mediated autoimmunity. Nat Commun. 2020. Sep;11(1):4859. 10.1038/s41467-020-18600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Trigo S, Blanco-Touriñán N, DeFalco TA, Wells ES, Gray JE, Zipfel C, et al. CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep. 2020. Feb;21(2):e48466. 10.15252/embr.201948466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang H, Feng B, Hu Z, Boisson-Dernier A, Franck CM, Meng X, et al. Differential regulation of two-tiered plant immunity and sexual reproduction by ANXUR receptor-like kinases. Plant Cell. 2017. Dec;29(12):3140–56. 10.1105/tpc.17.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Liu XX, Xie Y, Lin XY, Hu ZJ, Wang H, et al. Identification of FERONIA-like receptor genes involved in rice-Magnaporthe oryzae interaction. Phytopathol Res. 2020;2(1):14. 10.1186/s42483-020-00052-z [DOI] [Google Scholar]
- Yang Z, Xing J, Wang L, Liu Y, Qu J, Tan Y, et al. Mutations of two FERONIA-like receptor genes enhance rice blast resistance without growth penalty. J Exp Bot. 2020. Mar;71(6):2112–26. 10.1093/jxb/erz541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Li W, Luo X, Tang Y, Wang L, Yu F, et al. Rice FERONIA-LIKE RECEPTOR 3 and 14 affect grain quality by regulating redox homeostasis during endosperm development. J Exp Bot. 2023. May;74(10):3003–18. 10.1093/jxb/erad077 [DOI] [PubMed] [Google Scholar]
- Jing Y, Pei T, Li C, Wang D, Wang Q, Chen Y, et al. Overexpression of the FERONIA receptor kinase MdMRLK2 enhances apple cold tolerance. Plant J. 2023. Jul;115(1):236–52. 10.1111/tpj.16226 [DOI] [PubMed] [Google Scholar]
- Fan Y, Bai J, Wu S, Zhang M, Li J, Lin R, et al. The RALF2-FERONIA-MYB63 module orchestrates growth and defense in tomato roots. New Phytol. 2024. Aug;243(3):1123–36. 10.1111/nph.19865 [DOI] [PubMed] [Google Scholar]
- Ji D, Liu W, Cui X, Liu K, Liu Y, Huang X, et al. A receptor-like kinase SlFERL mediates immune responses of tomato to Botrytis cinerea by recognizing BcPG1 and fine-tuning MAPK signaling. New Phytol. 2023. Nov;240(3):1189–201. 10.1111/nph.19210 [DOI] [PubMed] [Google Scholar]
- Solís-Miranda J, Juárez-Verdayes MA, Nava N, Rosas P, Leija-Salas A, Cárdenas L, et al. The Phaseolus vulgaris Receptor-Like Kinase PvFER1 and the Small Peptides PvRALF1 and PvRALF6 Regulate Nodule Number as a Function of Nitrate Availability. Int J Mol Sci. 2023. Mar;24(6):5230. 10.3390/ijms24065230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Morales E, Jiménez-Chávez P, Olivares-Grajales JE, Sarmiento-López L, García-Niño WR, López-López A, et al. Role of a LORELEI- like gene from Phaseolus vulgaris during a mutualistic interaction with Rhizobium tropici. PLoS One. 2023. Dec;18(12):e0294334. 10.1371/journal.pone.0294334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LZ, Wang L, Chen X, Ge Z, Mergner J, Li X, et al. The RALF signaling pathway regulates cell wall integrity during pollen tube growth in maize. Plant Cell. 2024. May;36(5):1673–96. 10.1093/plcell/koad324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Li Y, Li Y, Ma H, Zuo C, Yang J, et al. GhRALFs and GhMLDRLKs synergize to confer thermotolerance in cotton. 2025. Submitted.
- Masachis S, Segorbe D, Turrà D, Leon-Ruiz M, Fürst U, El Ghalid M, et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat Microbiol. 2016. Apr;1(6):16043. 10.1038/nmicrobiol.2016.43 [DOI] [PubMed] [Google Scholar]
- Thynne E, Saur IM, Simbaqueba J, Ogilvie HA, Gonzalez-Cendales Y, Mead O, et al. Fungal phytopathogens encode functional homologues of plant rapid alkalinization factor (RALF) peptides. Mol Plant Pathol. 2017. Aug;18(6):811–24. 10.1111/mpp.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang D, Chen J, Wu D, Feng X, Yu F. Nematode RALF-like1 targets soybean malectin-like receptor kinase to facilitate parasitism. Front Plant Sci. 2021. Dec;12:775508. 10.3389/fpls.2021.775508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu X, Yuan B, Chen X, Zhao H, Ali Q, et al. Fusarium graminearum rapid alkalinization factor peptide negatively regulates plant immunity and cell growth via the FERONIA receptor kinase. Plant Biotechnol J. 2024. Jul;22(7):1800–11. 10.1111/pbi.14303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Salt-induced root cell responses. Top, a wild-type root; Bottom, a fer-4 root.
RALF1 induced FER-GFP clustering and endocytosis.
RALF1 induced GFP-LLG1 clustering and endocytosis.